41 lewis diagram for bf3
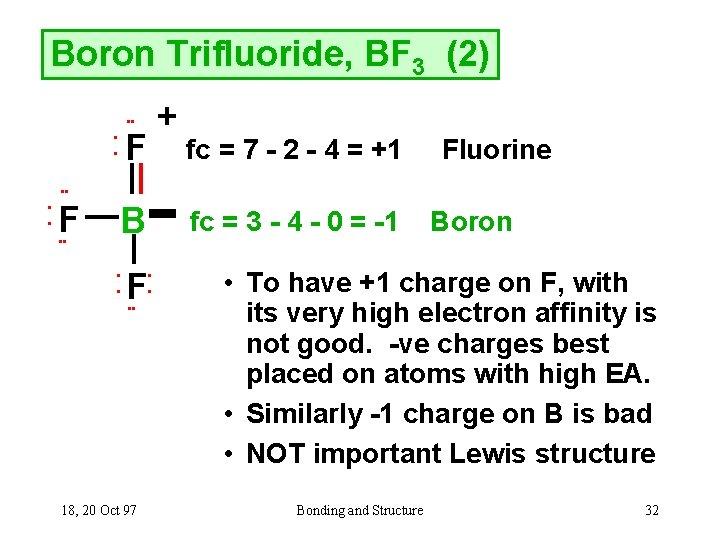
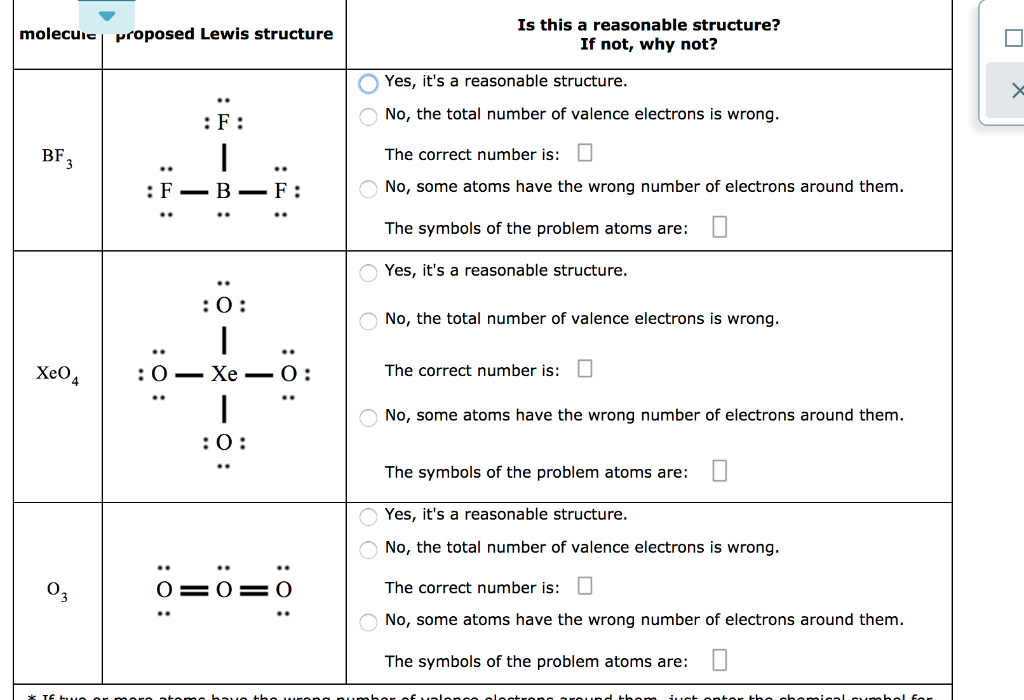
Boron trifluoride is a versatile Lewis acid that forms adducts with such Lewis bases as fluoride and ethers: CsF + BF 3 → CsBF 4 O(C 2 H 5) 2 + BF 3 → BF 3 ·O(C 2 H 5) 2. Tetrafluoroborate salts are commonly employed as non-coordinating anions. There are a total of 24 valence electrons for the BF3 Lewis structure. After determining how many valence electrons there are in BF3, place them around the central atom to complete the octets. Boron is the least electronegative atom in the BF3 Lewis structure and therefore goes at the center of the structure.
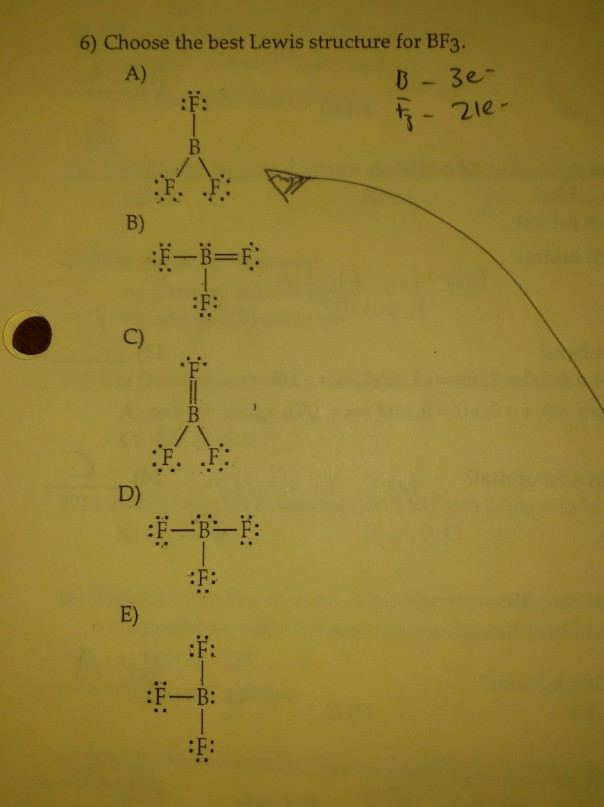
Answer to: Draw the Lewis dot structure for BF3 and provide the following information. a. number of bond pairs b. number of lone pairs c. molecular...
Lewis diagram for bf3
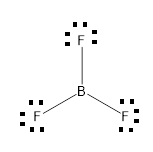
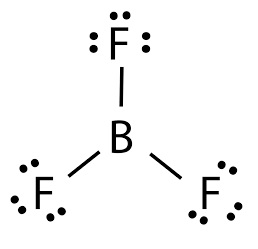
Lewis structure of boron trifluoride (BF 3) is shown below and you can see each fluorine atom has made a single bond with boron atom. Boron atom is the center atom. Boron atom is the center atom. In this tutorial, we will learn how to draw the lewis structure of BF 3 with all theories. If you look at the Lewis Structure for BF3 it appears to be a symmetrical molecule. However, to determine if BF3 is polar we consider the molecular geometry. A polar molecule results from an unequal/unsymmetrical sharing of valence electrons. For Boron trifluoride the molecule is symmetrical and therefore it is a nonpolar molecule. Drawing the Lewis Structure for BF 3. Viewing Notes: The BF 3 Lewis structure is similar to BCl 3 and BBr 3 since Cl and Br are in Group 7 and have 7 valence electrons.; Boron (B) doesn't need 8 valence electrons to have an octet (Boron often only needs 6). If you're not sure you have the best Lewis structure for BF 3 you can calculate the formal charges. You'll find the B in BF 3 only has 6 ...
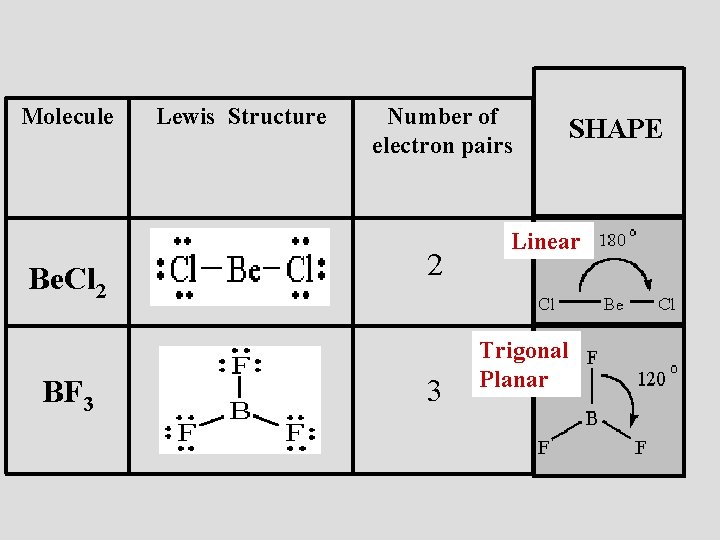
Lewis diagram for bf3. Step 1: Use lewis structure guidelines to draw the lewis structure of BF 3. Step2: Apply VSEPR notation, A X E A=Number of central atoms X=Number of surrounding atoms E= Number of lone pairs on central atom For the above molecule VSEPR notation will be AX 3 E 0. Step 3: Use VSEPR table to find the shape. AX 3 has trigonal planar shape. What is Lewis structure of bf3? There are a total of 24 valence electrons for the BF3 Lewis structure. After determining how many valence electrons there are in BF3, place them around the central atom to complete the octets. Boron is the least electronegative atom in the BF3 Lewis structure and therefore goes at the center of the structure. normally lewis acids are species with vacant orbitals,while lewis bases are species with lone pair of electron , in the given reaction the BF3 has a vacant orbital ( draw the lewis structure of BF3) while F- has the lone pair of electron which it donates to the BF 3. . Answer. BF3 is acting as lewis acid since it accepts a pair of electrons. Topic. Lewis Diagram and Molecular Shape of Nitrogen trifluoride NF3 Lewis Diagrams and Molecular Shape of Boron trifluoride BF3 Be sure to subscribe to my Youtube Channel!
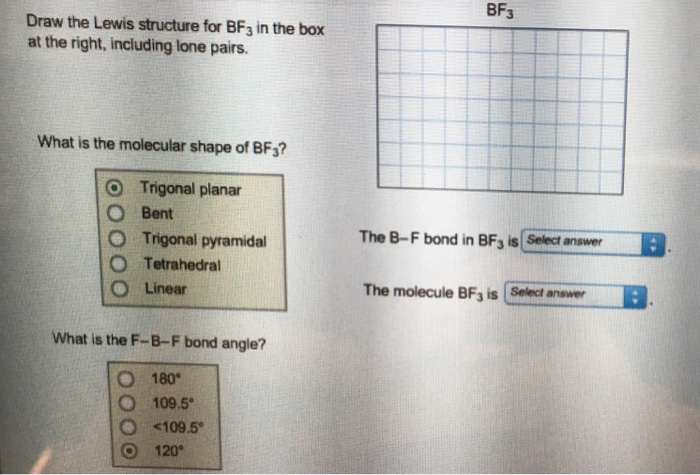
What is the Lewis Structure for BF3, the steric number, the electron pair geometry, the molecular geometry, and how many double bonds does it have? close. Start your trial now! First week only $4.99! arrow_forward. Question. A step-by-step explanation of how to draw the BF3 Lewis Dot Structure (Boron Trifluoride).For the BF3 Lewis structure, calculate the total number of valence ... Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra... When NH3 contributes its lone pair of electrons to BF3, it serves as a Lewis base in this diagram. When BF3 absorbs the lone pair of electrons that NH3 donates, it becomes a Lewis acid. This reaction fills BF3's vacant 2p-orbital, making boron sp3 hybridize where it was previously sp2 hybridized (as BF3).
Boron trifluoride is a colorless gas with a pungent odor. It is toxic by inhalation. It is soluble in water and slowly hydrolyzed by cold water to give off hydrofluoric acid, a corrosive material.Its vapors are heavier than air. Prolonged exposure of the containers to fire or heat may result in their violent rupturing and rocketing. The SF4 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the SF4 molecule. The geometry of the SF4 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory (VSEPR Theory), which states that molecules will choose the SF4 geometrical shape in which the electrons ... BF3 Lewis Structure To know the structure BF3 Lewis, we need to calculate the total number of valence electrons for the BF3 molecule. BF3 has a total of 24 valence electrons, we must set around the central atom. Before completing octets, do not forget to determine how many valence electrons there in Boron trifluoride and place them accordingly. Drawing the Lewis Structure for BF 3. Video: Drawing the Lewis Structure for BF 3. For the BF 3 Lewis structure, calculate the total number of valence electrons for the BF 3 molecule. There are a total of 24 valence electrons for the BF 3 Lewis structure. After determining how many valence electrons there are in BF 3, place them around the central atom to complete the octets.
Hydrogen is usually surrounded by 4 electrons in a valid lewis structure. Bf3 has a total of 24 valence electrons, which we have to set around the central atom. Lewis structure, then the molecular geometry of the molecules. Ax 2 has linear shape. Predicted data is generated using the us environmental protection agency's episuite™.
Drawing the Lewis Structure for BrF 3. Video: Drawing the Lewis Structure for BrF 3. For the BrF 3 Lewis structure, calculate the total number of valence electrons for the BrF 3 molecule. There are a total of 28 valence electrons for the BrF 3 Lewis structure. After determining how many valence electrons there are in BrF 3, place them around the central atom to complete the octets.
The bonds that are formed between two. The answer is A square planar. Start from the Lewis structure of the tetrafluoroborate ion BrF 4. A step-by-step explanation of how to draw the BF3 Lewis Dot Structure Boron TrifluorideFor the BF3 Lewis structure calculate the total number of valence.
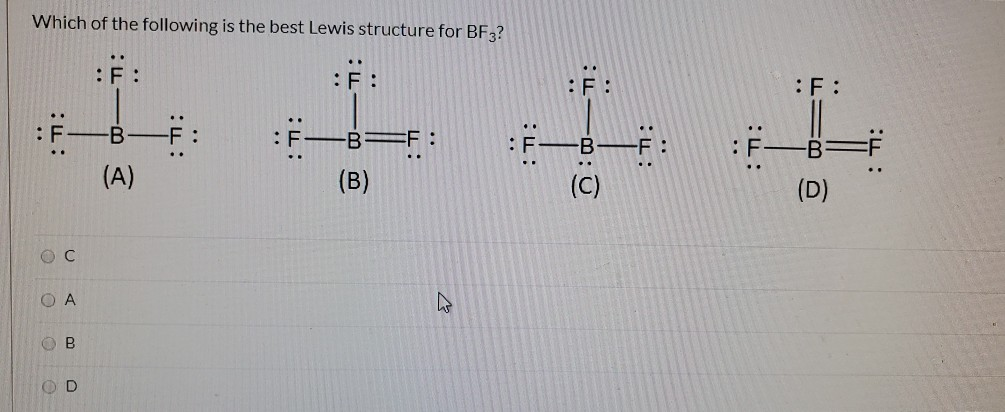
Problem: For the molecule BF3, if we write the Lewis dot structure by first completing the octet around the pendant F atoms, we find that we have used all the valence electrons but the B has less than an octet (Structure A). Using our rules for drawing Lewis dot structures, we complete the octet around the B by forming double bonds from one of the F atoms which give rise to the resonance ...
Check me out: http://www.chemistnate.com
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
The Lewis diagram for BF_3 is: The electron-pair geometry around the B atom in BF_3 is There are lone pair(s) around the central atom, so the geometry of BF_3 is The Lewis diagram for BeF_2 is: The electron-pair geometry around the Be atom in BeF_2 is There are lone pairs around the central atom. so the geometry of Be_F_2, is
What is the Lewis dot structure of BF3? There are a total of 24 valence electrons for the BF3 Lewis structure. After determining how many valence electrons there are in BF3, place them around the central atom to complete the octets. Boron is the least electronegative atom in the BF3 Lewis structure and therefore goes at the center of the structure.

Draw The Lewis Structure For Bf3 How Many Bonds Are Around The Central Atom And What Is The Shape Of This Molecule Study Com
BF3, boron trifluoride, is a tricky molecule to draw because Boron is an exception to the octet rule.It does not need eight electrons in its outer shell, although it can hold eight just like most other non-metals.. The Lewis Structure of BF3, boron trifluoride, has one boron atom in the centre, and three fluorine atoms surrounding it.
BF3 Lewis Structure. To know about BF3 Lewis structure, we have to calculate the total number of valence electrons for the BF3 molecule. BF3 has a total of 24 valence electrons, which we have to set around the central atom.
BF3 Lewis Structure, Molecular Geometry, and Hybridization. Boron Trifluoride (BF3) is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule. Manufactured from the reaction of boron oxides and hydrogen fluoride, the chemical compound BF3 has a pungent smell and is colorless in nature. The compound behaves differently in ...
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...

Draw The Lewis Dot Structure For Bf3 And Provide The Following Information A Number Of Bond Pairs B Number Of Lone Pairs C Molecular Geometry D Hybridization Of The Central Atom
In BF3 (Boron Trifluoride) molecule there are three bonds of B-F, as shown clearly in Lewis diagram above. The electronegativity of B (Boron) is 2.04 and of F (Fluorine) is 3.98 (maximum) according to the Pauling scale which means that F (Fluorine) will pull the shared electrons towards itself and will thus acquire a partial negative charge (δ ...
Drawing the Lewis Structure for BF 3. Viewing Notes: The BF 3 Lewis structure is similar to BCl 3 and BBr 3 since Cl and Br are in Group 7 and have 7 valence electrons.; Boron (B) doesn't need 8 valence electrons to have an octet (Boron often only needs 6). If you're not sure you have the best Lewis structure for BF 3 you can calculate the formal charges. You'll find the B in BF 3 only has 6 ...
If you look at the Lewis Structure for BF3 it appears to be a symmetrical molecule. However, to determine if BF3 is polar we consider the molecular geometry. A polar molecule results from an unequal/unsymmetrical sharing of valence electrons. For Boron trifluoride the molecule is symmetrical and therefore it is a nonpolar molecule.
Lewis structure of boron trifluoride (BF 3) is shown below and you can see each fluorine atom has made a single bond with boron atom. Boron atom is the center atom. Boron atom is the center atom. In this tutorial, we will learn how to draw the lewis structure of BF 3 with all theories.








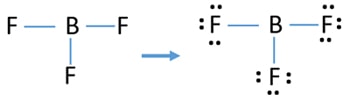










0 Response to "41 lewis diagram for bf3"
Post a Comment