45 pv diagram problems and solutions
EXAMPLE PROBLEMS AND SOLUTIONS A-3-1. Simplify the block diagram shown in Figure 3-42. Solution. First, move the branch point of the path involving HI outside the loop involving H,, as shown in Figure 3-43(a). Then eliminating two loops results in Figure 3-43(b). Combining two blocks into one gives Figure 3-33(c). A-3-2. First law of thermodynamics problem solving. PV diagrams - part 1: Work and isobaric processes. PV diagrams - part 2: Isothermal, isometric, adiabatic processes. Second law of thermodynamics. Next lesson. Thermochemistry.
6:05Topic covered:- calculation of work done on pv graph problems.easy method to solve themodynamics part :-13 ...2 Jan 2017 · Uploaded by VEDANTU NEET MADE EJEE
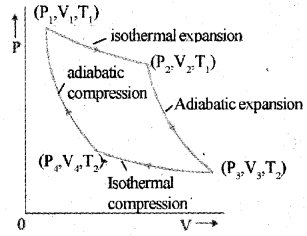
Pv diagram problems and solutions
A cyclic Process ABCA as shown in below V-T diagram is performed with a constant mass of ideal gas.Show the process in the P-V digram. Solution. A straight line between A to B on V-T diagram indicates V αT V α T .So Pressure is constant. Volume is constant from B-C .Now since temperature is decreasing, Pressure must decrease. Isothermal thermodynamic processes – problems and solutions. 1. PV diagram below shows an ideal gas undergoes an isothermal process. Calculate the work is done by the gas in the process AB. Solution. Work done by a gas is equal to the area under the PV curve. AB = triangle area + rectangle area. W = [½ (8 x 10 5 –4 x 10 5)(3-1)] + [4 x 10 ... Problems practice. One mole of an ideal, monatomic gas runs through a four step cycle. All processes are either isobaric or isochoric. The pressure and volume of the gas at the extreme points in the cycle are given in the table below. Sketch the PV graph of this cycle. Determine the temperature at state A, B, C, and D.
Pv diagram problems and solutions. ACT 1: Solution Consider the two systems shown to the right. In Case I, the gas is heated at constant volume ; in Case II, the gas is heated at constant pressure . Compare Q I, the amount of heat needed to raise the temperature 1ºC in system I to Q II, the amount of heat needed to raise the temperature 1ºC in system II. A) Q I< Q II B) Q I= Q ... 10 Sep 2018 — Cyclic processes · The PV diagrams for a thermodynamical system is given in the figure below. · In the case (a) the closed curve is anticlockwise. look at PV diagrams. A PV diagram is a graph of Pressure as a function of Volume. There are four different situations that you can expect to see shown in PV diagrams: 1. Isobaric: the gas is held at a constant pressure 2. Isochoric: the gas is held at a constant volume 3. Isothermal: the gas is held at a constant temperature 4. Sketch the cycle path on a PV Diagram b.) Calculate the net work done in kJ Data: T 1 = 145 o C, T 2 = 440 o C, P 1 = 150 kPa : Read : Work your way around the cycle, step by step. The work for the cycle is the sum of the work for each step. Assume the CO 2 behaves as an ideal gas throughout all three process steps.
Problem #6: A 12.0 g sample of gas occupies 19.2 L at STP. What is the molecular weight of this gas? Solution: This problem, as well as the two just above can be solved with PV = nRT. You would solve for n, the number of moles. Then you would divide the grams given by the mole calculated. 1) Use PV = nRT: (1.00 atm) (19.2 L) = (n) (0.08206) (273 K) Solar system problems may have complex causes, but solving them is usually routine. It just takes experience to get started off on the right foot. Keeping track of your system’s performance is an easy, effective troubleshooting solution. So is staying in touch with a specialist. Problems practice. One mole of an ideal, monatomic gas runs through a four step cycle. All processes are either isobaric or isochoric. The pressure and volume of the gas at the extreme points in the cycle are given in the table below. Sketch the PV graph of this cycle. Determine the temperature at state A, B, C, and D. Isothermal thermodynamic processes – problems and solutions. 1. PV diagram below shows an ideal gas undergoes an isothermal process. Calculate the work is done by the gas in the process AB. Solution. Work done by a gas is equal to the area under the PV curve. AB = triangle area + rectangle area. W = [½ (8 x 10 5 –4 x 10 5)(3-1)] + [4 x 10 ...
A cyclic Process ABCA as shown in below V-T diagram is performed with a constant mass of ideal gas.Show the process in the P-V digram. Solution. A straight line between A to B on V-T diagram indicates V αT V α T .So Pressure is constant. Volume is constant from B-C .Now since temperature is decreasing, Pressure must decrease.




0 Response to "45 pv diagram problems and solutions"
Post a Comment