45 bohr diagram for carbon
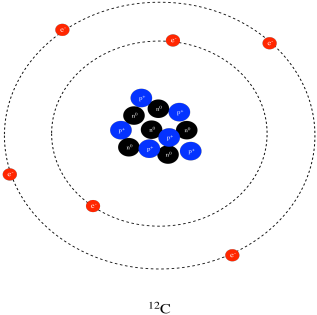
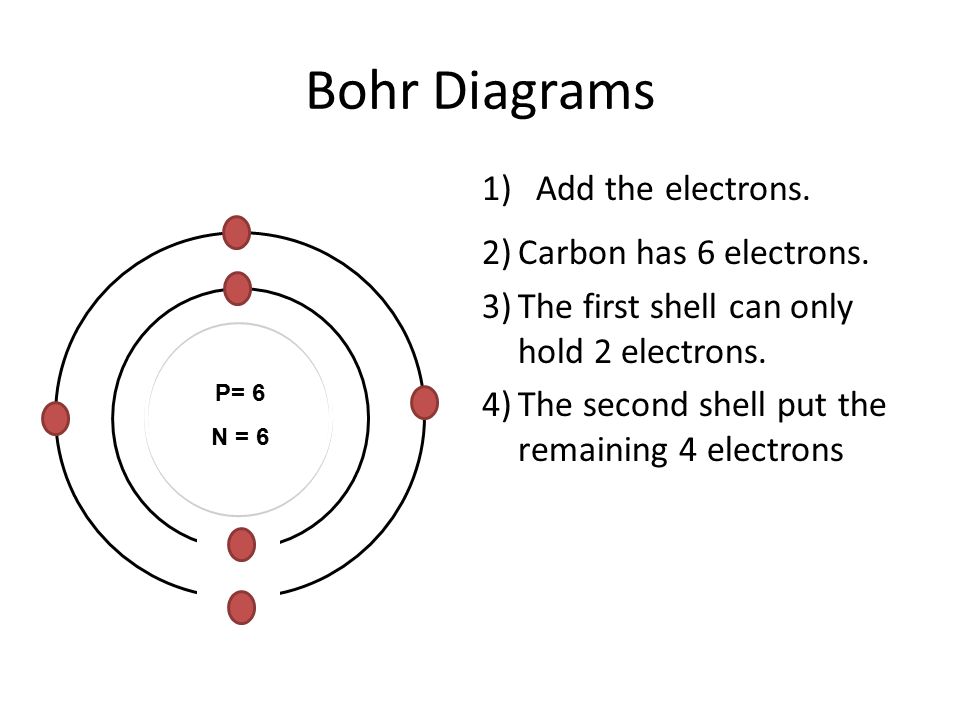
Use the diagram to the right to answer question 1. _____1. The atomic number of carbon is 6, which means that carbon atoms always have 6 A. ions B. protons C. neutrons D. valence electrons _____2. In his investigations of air, Henry Cavendish discovered a small bubble of leftover gas that would not combine with nitrogen. Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n
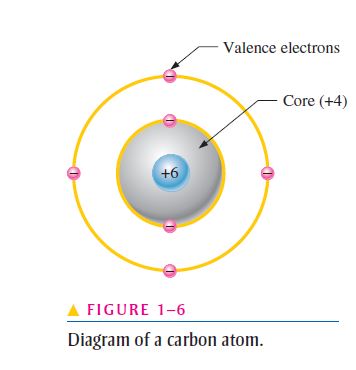
A Lewis diagram is a diagram that only shows the valence electrons of an atom and does not show the different shells an atom has like a Bohr diagram does. Carbon has four valence electrons and the Lewis diagram of carbon can be seen on the right.
Bohr diagram for carbon
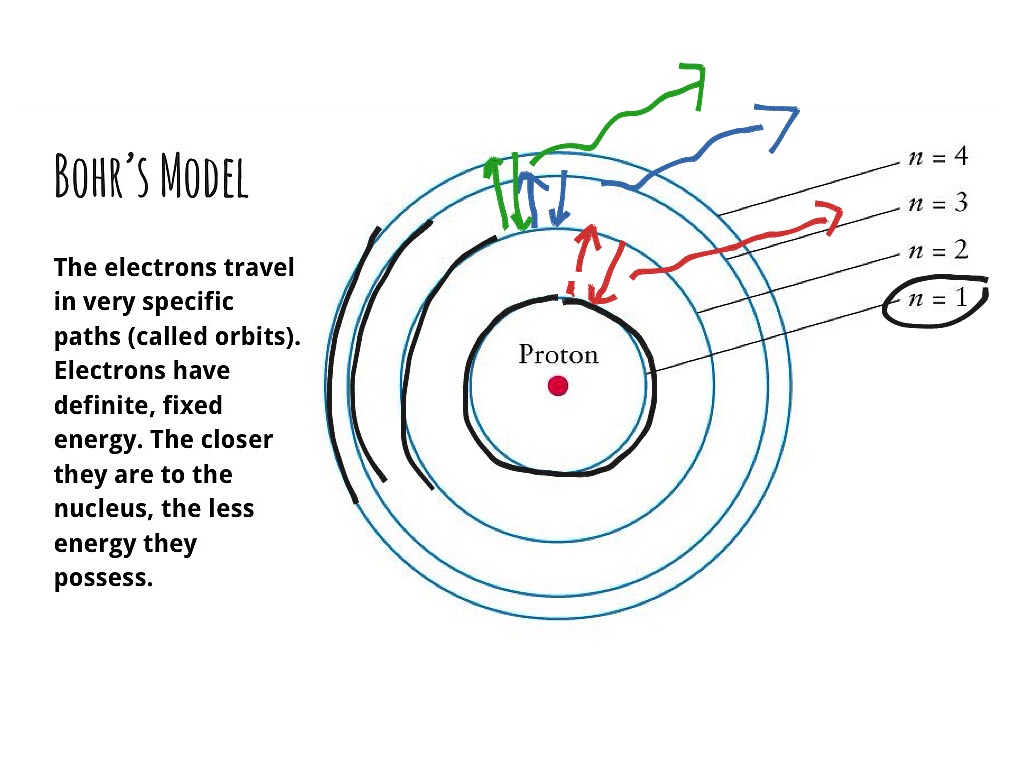
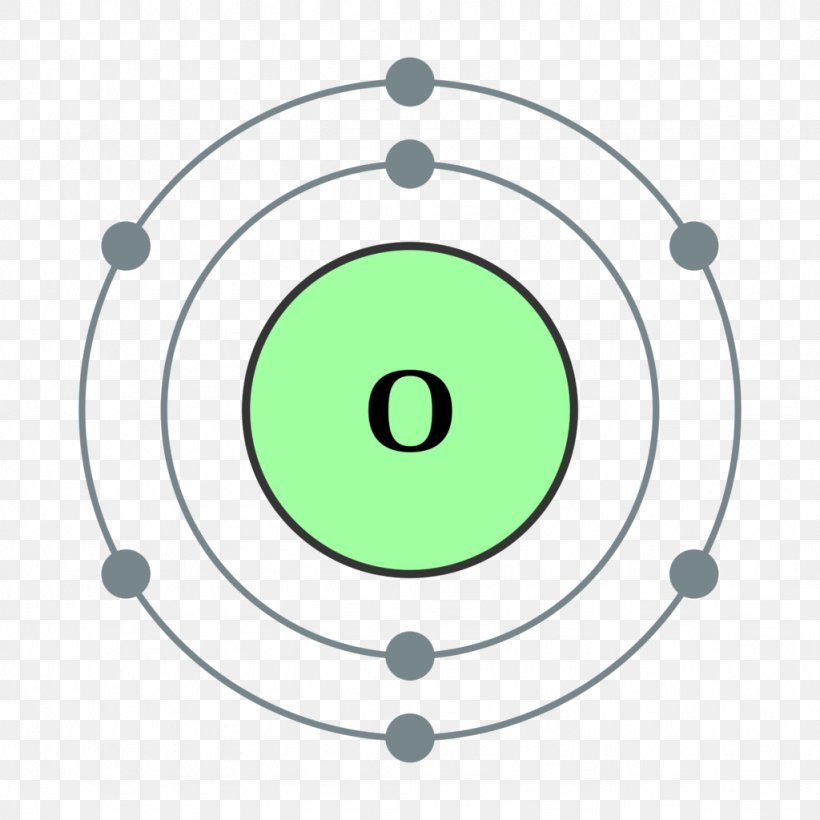
According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons. The Bohr effect is a phenomenon first described in 1904 by the Danish physiologist Christian Bohr. Hemoglobin's oxygen binding affinity (see oxygen-haemoglobin dissociation curve) is inversely related both to acidity and to the concentration of carbon dioxide. That is, the Bohr effect refers to the shift in the oxygen dissociation curve caused by changes in the concentration of carbon ... Bohr diagram for carbon. Wiki User. ∙ 2012-10-12 14:51:55. See Answer. Best Answer. Copy. i really dont knw at all i came here to figure it out but i guess nobody knws to help me so if u came to ...
Bohr diagram for carbon. Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons Bohr Diagrams. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. Carbon dioxide transport Atomic physics Bohr model of the atom. by crator-avatar. An example of a covalent compound is H2O, its correct Bohr But how can I draw the Bohr diagram for N2 and CO2? I attempted to draw the. most-viewed-thumbnail. Carbon Bohr model most-viewed-thumbnail. Carbon dioxide transport Atomic physics Bohr model of the atom ... How to draw bohr diagrams michelle bartels bohr diagrams 1 draw a nucleus with the number of protons and neutrons inside 2 carbon is in the 2nd period so it has two energy levels or shells. This lesson will walk your students through the basics on how to draw a bohr diagram for the first 20 elements on the periodic table. 2 carbon has 6 ...
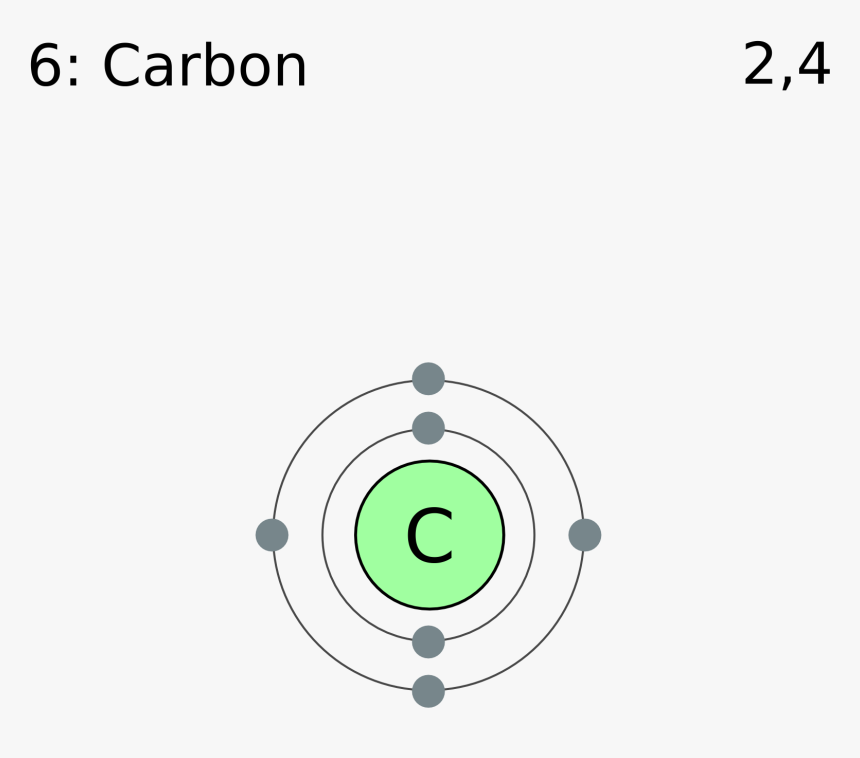
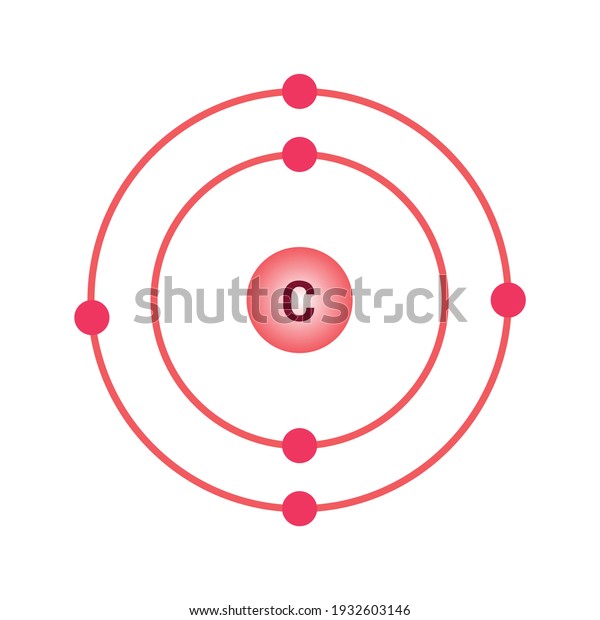
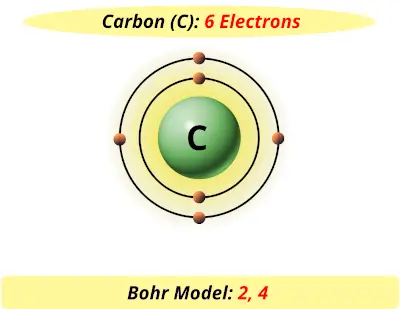
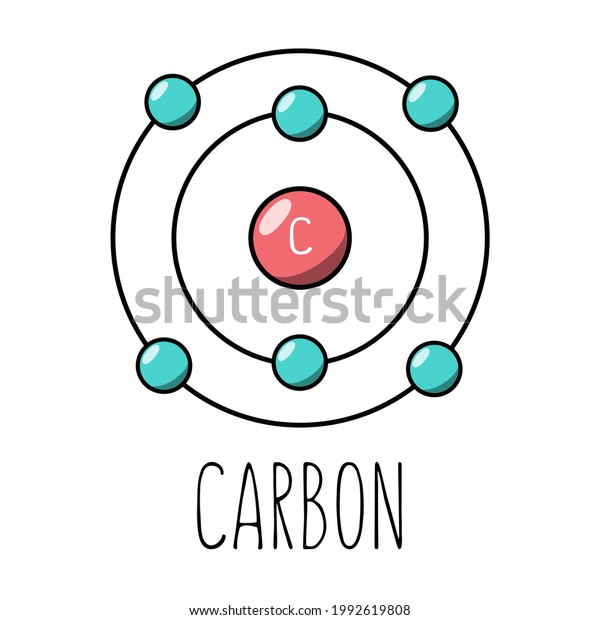
A step by step program for creating Bohr Diagrams and Electron dot structures. Usefule for Index Cards and Flash cards for chemistry and physical science stude… SlideShare uses cookies to improve functionality and performance, and to provide you with relevant advertising. Bohr Model of Hydrogen. The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. The Bohr model of Carbon is drawn with only two electron shells, the first shell contains 2 electrons and the second shell contains 4 electrons. Carbon is neutral and its atomic number is 6, hence, the number of protons and electrons available for its Bohr diagram is also 6. The number of neutrons for the Bohr diagram of Carbon can be found by ... Carbon has 2 electrons in its first shell and 4 in its second shell.Check me out: http://www.chemistnate.com
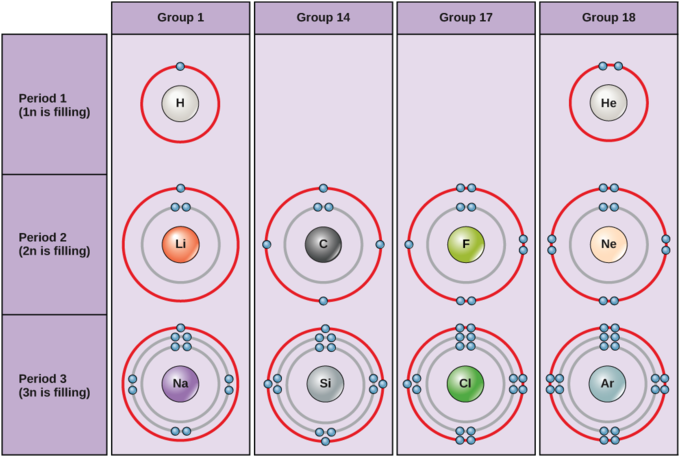
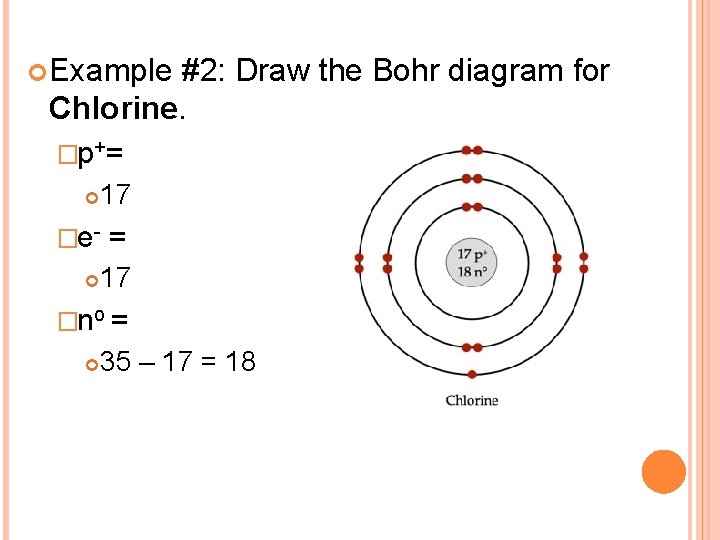
HOW TO DRAW BOHR DIAGRAMS - In this video, I'll teach you how to draw bohr diagrams for carbon (C), sodium (Na), and phosphorous (P). The steps to drawing bo... Bohr Diagrams 1) Draw a nucleus with the element symbol inside. 2) Carbon is in the 2nd C period, so it has two energy levels, or shells. 3) Draw the shells around the nucleus. 8. Bohr Diagrams 1) Add the electrons. 2) Carbon has 6 electrons. C 3) The first shell can only hold 2 electrons. 9. Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st energy level. 4) The 2nd energy level can hold up to 8 electrons. 5) The 3rd energy level can hold 18, but the outer shell can only hold 8 electrons. C P+ = 6 N0 = 6 2e-4e- Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of ...
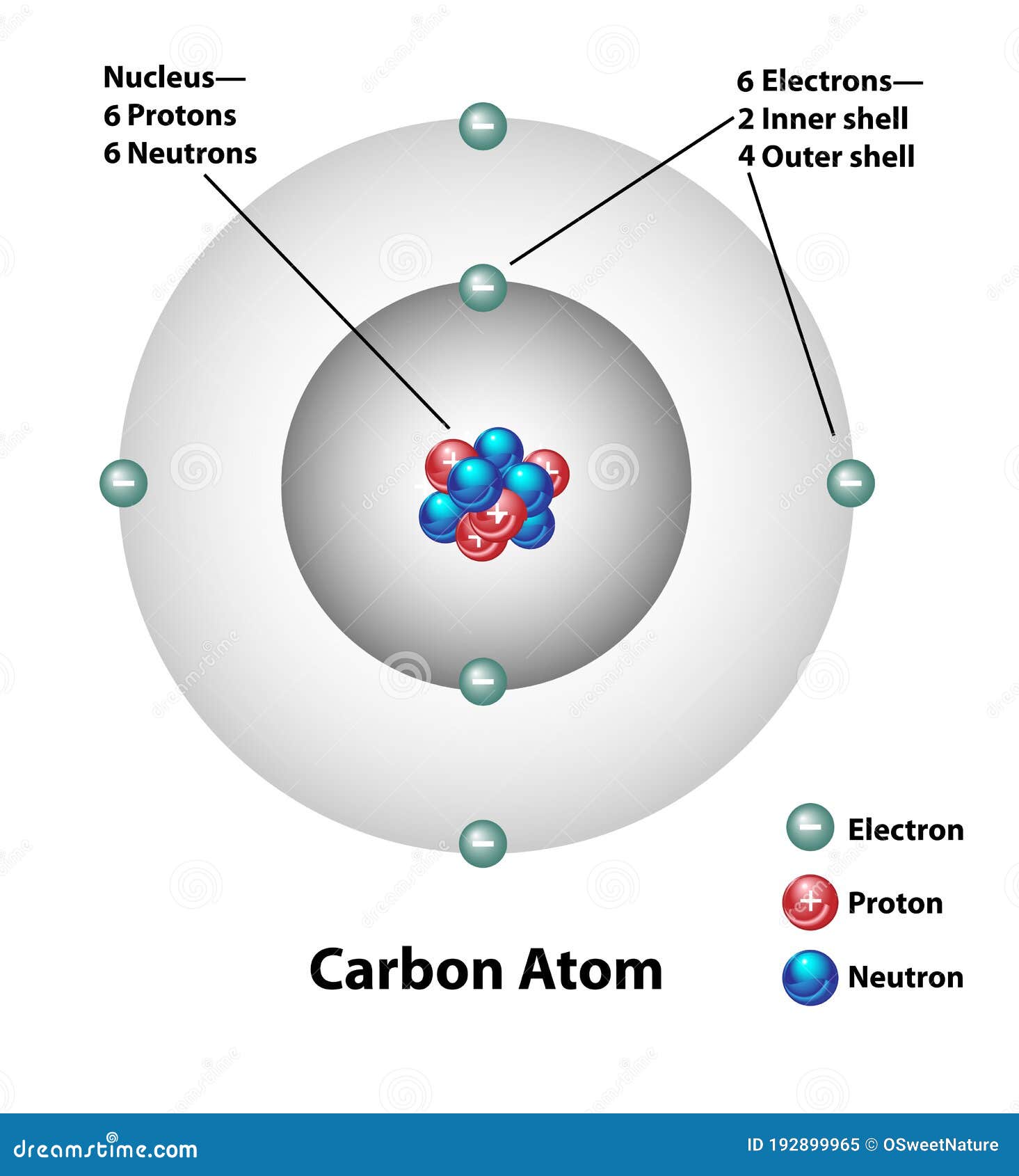
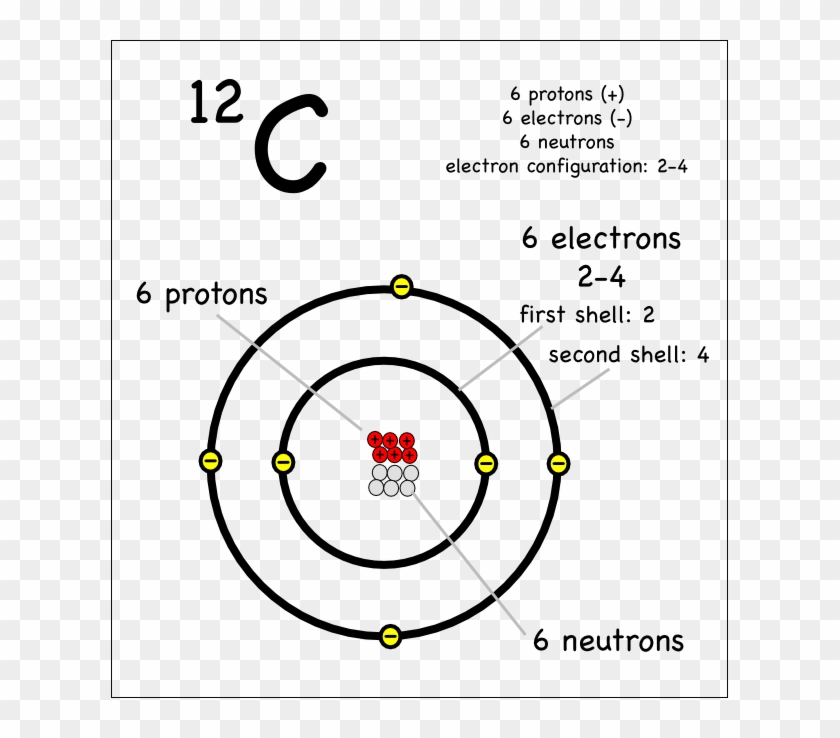
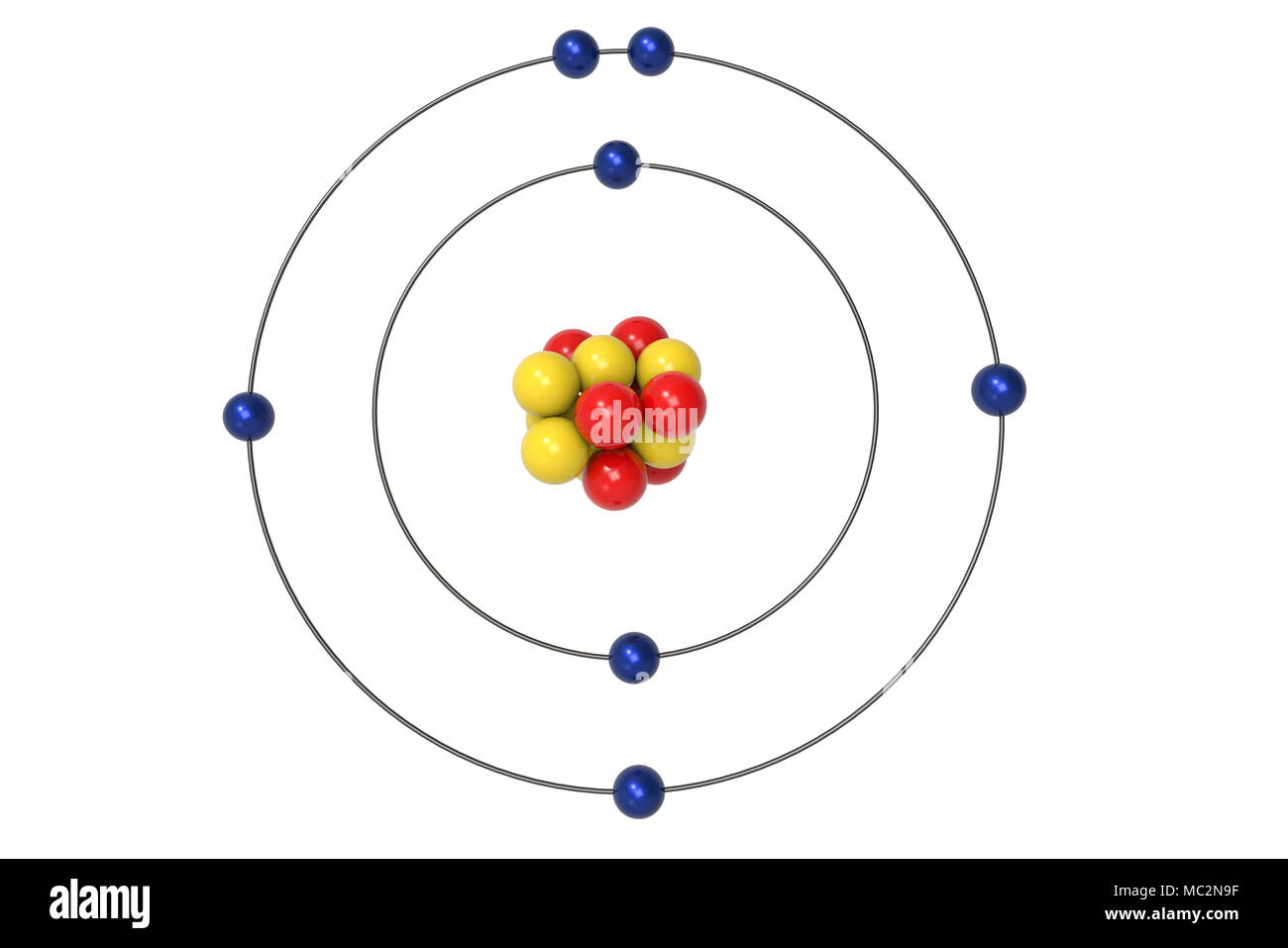
Name: Carbon Symbol: C Atomic Number: 6. Atomic Mass: 12.0107 amu ... Number of Protons/Electrons: 6. Number of Neutrons: 6 ... [Bohr Model of Carbon]
Herein, what is the Bohr diagram for carbon? Bohr Diagrams 1) Add the electrons.2) Carbon has 6 electrons.C 3) The first shell can only hold 2 electrons.The 2nd shell can hold up to 8 electrons.. Also Know, what is the number of protons neutrons and electrons in carbon?
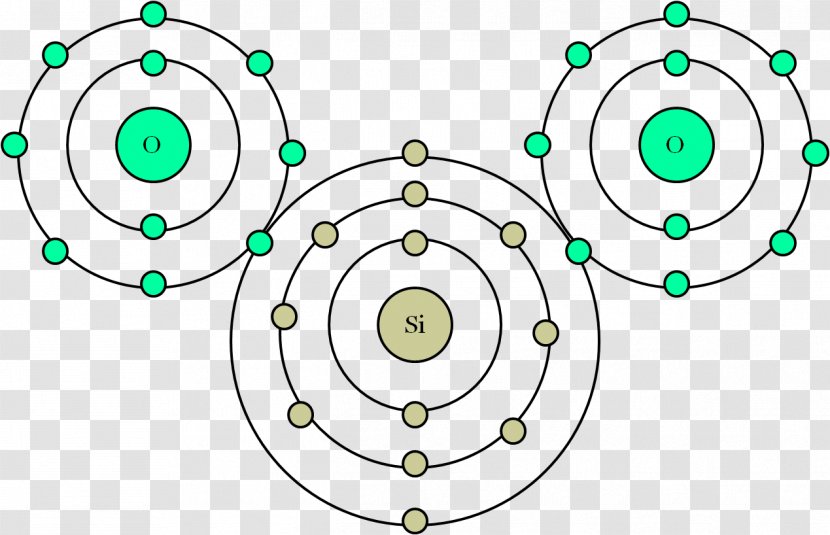
Carbon is a non-metal which has 4 valence electrons (that means 4 electrons in its outer shell, the second).Oxygen is also a non-metal, with 6 electrons in i...
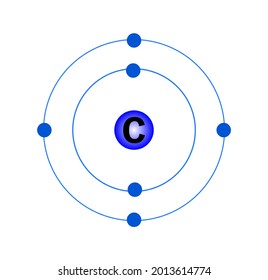
The Bohr model of carbon has a central nucleus containing six protons and six neutrons, encircled by an inner orbit of two electrons and an outer orbit of four electrons. The two orbits represent different energy levels and are at a set distance from one another and from the nucleus. In the Bohr model, electrons with less energy occupy orbits ...
Nitrogen has 2 electrons in its first shell and 5 in its second.Check me out: http://www.chemistnate.com
Calcium has 2 electrons in its first shell, 8 in its second, 8 in its third, and 2 in its fourth.Check me out: http://www.chemistnate.com
Aluminum has 2 electrons in its first shell, 8 in its second and 3 in its third.Check me out: http://www.chemistnate.com
The Bohr Model of Magnesium(Mg) has a nucleus that contains 12 neutrons and 12 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Magnesium contains 2 electrons that also called valence electrons.
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n
What is the Bohr model for carbon? A Bohr model is a way of visually depicting the structure of an atom, specifically to show the location of its electrons in their energy levels. The Bohr model shows that the protons and neutrons are located in the nucleus, while electrons orbit in energy levels. Subsequently, one may also ask, what does Bohr ...
How to make a Bohr model of carbon A Visual Depiction: A Bohr model is a way of visually depicting the structure of an atom, specifically to show the location of its electrons in their energy levels.
Bohr diagram for carbon. Wiki User. ∙ 2012-10-12 14:51:55. See Answer. Best Answer. Copy. i really dont knw at all i came here to figure it out but i guess nobody knws to help me so if u came to ...
The Bohr effect is a phenomenon first described in 1904 by the Danish physiologist Christian Bohr. Hemoglobin's oxygen binding affinity (see oxygen-haemoglobin dissociation curve) is inversely related both to acidity and to the concentration of carbon dioxide. That is, the Bohr effect refers to the shift in the oxygen dissociation curve caused by changes in the concentration of carbon ...
According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons.



















0 Response to "45 bohr diagram for carbon"
Post a Comment