41 molecular orbital diagram problems
www.calgarylearning.ca Hi, I am a recent PhD grad (Chemical Engineering. Over the past seven years I have served as a tutor, teaching assistant, lecturer, and lab instructor in a number of courses for high school and engineering students. My long term goals are towards Professorship and I am hence, offering tutoring to students for a number of University Mathematics, Physics Chemistry and Stats courses. These include courses as Kinematics, Work Energy Power, Rotation in Physics, Chemical Eq... As a science communicator, I'm very interested in raising the science literacy of the general public. That's why I've created a series of general chemistry tutorials that cover the topics in a year of high school chemistry, but in as clear and concise a way as possible. There are quantitative practice problems for those who want to learn the math, but it's also for any person to simply watch in a relaxed way in order to absorb the content and expand their understanding. I genuinely believe it's ...
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...

Molecular orbital diagram problems
Topic: Combination of two atomic orbitals results in the formation of two molecular orbitals namely _____ a one bonding and one non-bonding orbital b two bonding orbitals c two non-bonding orbitals d two bonding and non-bonding orbitals.Draw The Molecular Orbital Diagram Of N2n2 N2 Write Class 11 Chemistry Cbse Questions On Molecular Orbital Theory Class 11 This may be compared to a total of 20 spin orbitals in the simulation of the entire molecule, which shows a large reduction in the problem size: a 20-qubit problem is reduced to 10 two-qubit problems. "relating to or consisting of molecules," by 1815, from molecule + -ar or else from French moléculaire or Modern Latin molecularis. Molecular biology is attested by 1950.
Molecular orbital diagram problems. A little background: I am in my junior year of college working towards my BS in chemistry and hopefully moving on to grad school. I've taken two organic chemistry classes, one organic lab class (the higher lab class for chemistry majors vs the normal orgo lab class for non chemistry majors). I've taken one inorganic chemistry class and am currently in a class called advanced inorganic. I am also currently taking an inorganic lab class. I've also taken my general chemistry classes, analytical, an... The above frontier molecular orbital diagram becomes more intriguing on moving over to the metallocenes that contain two such cyclopentadienyl ligands. Specifically, in the Cp 2 M system, ( e. g. ferrocene) each of these above five molecular orbital of the two cyclopentadienyl ligands combines to give ten ligand molecular orbitals in three ... 1918 (Venn's diagram is from 1904), named for English logician John Venn (1834-1923) of Cambridge, who explained them in the book "Symbolic Logic" (1881). Whereas molecular geometry considers only the atoms. In absence of a lone pair, both the geometries are the same for any compound. Below is the 3D view of the geometry of the SO2 molecule. SO2 Geometry. Now let’s learn the last topic of this article, the molecular orbital diagram of SO2. SO2 Molecular Orbital Diagram
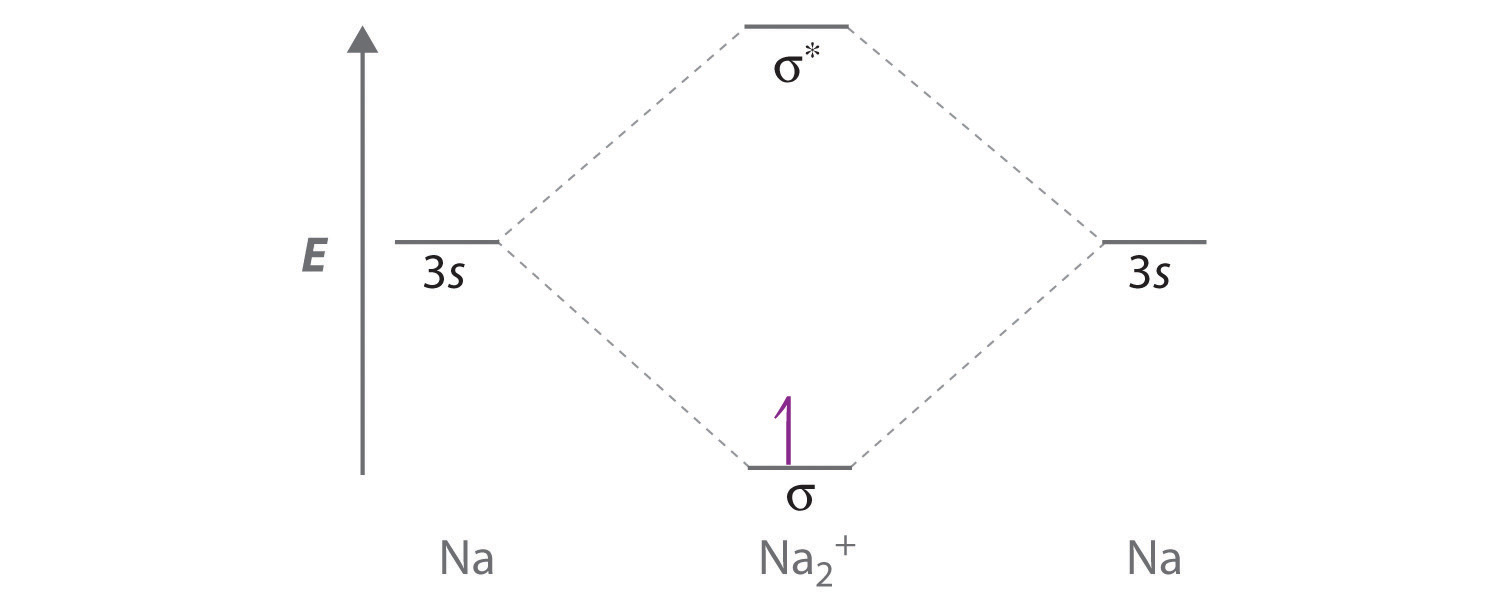
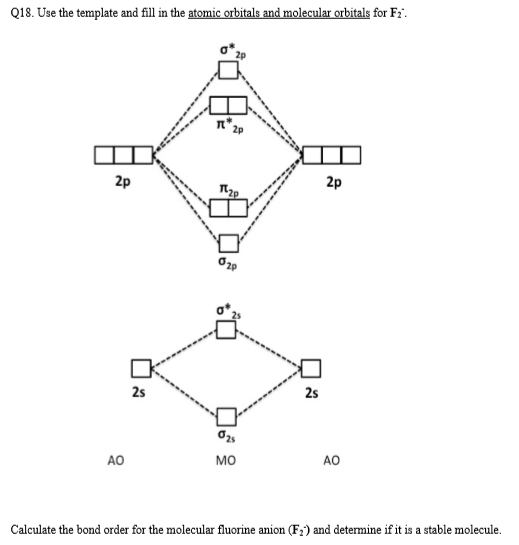
Of these options, F⁺ has the electron configuration [He]2s²2p⁴, which has unpaired electrons in the 2p orbital. Therefore, F⁺ will be paramagnetic and therefore attracted to a magnetic field. Use the MO diagram (below) to calculate the bond order for I₂. The d electron count is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex. The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes. The formalism has been incorporated into the two major models used to describe coordination … In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital. The bonding MO is occupied by two electrons of opposite spin, the result being a covalent bond. Bonding molecular orbitals are formed by in-phase combinations of atomic wave functions, and electrons in these orbitals stabilize a molecule. Antibonding molecular orbitals result from out-of-phase combinations of atomic wave functions and electrons in these orbitals make a molecule less stable. This made me think about two problems:
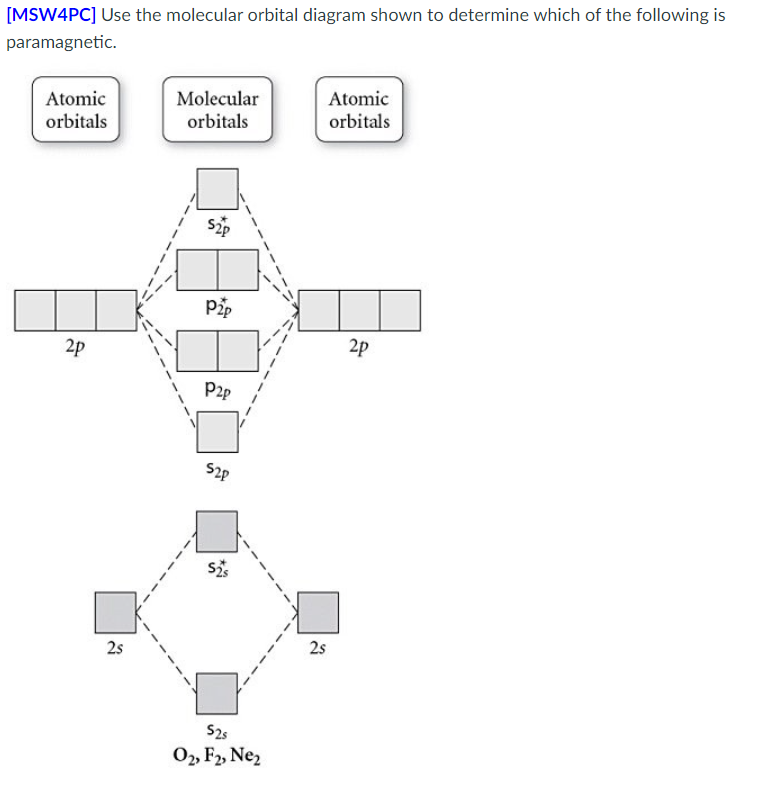
I have a homework problem asking me to construct the molecular orbital diagram for methylene chloride, and I am not too sure what to do next. I have determined that all of the orbitals transform as follow: C 2S=A1 C 2Pz=A1 C 2Py=B2 C 2Px=B1 2H 1S=A1+B2 2Cl 3S=A1+B1 2Cl 3Pz=A1+B1 2Cl 3Py=A2+B2 2Cl 3Px=A1+B1 My thoughts were to construct the MO for the CH2 side, then add the two chlorines from there. Let me know if you have any pointers. thank you This problem, and many others, can be overcome by using a more ... Diagram. One of these orbitals is called a bonding molecular orbital because electrons in ... Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B_2^2+, B2, C_2^2-, B_2^2- and N_2^2+ b. Draw the Lewis structures and molecular orbital diagrams for 1610s, "an illustrative figure giving only the outlines or general scheme of the object;" 1640s in geometry, "a drawing for the purpose of demonstrating the properties of a figure;" from French diagramme, from Latin diagramma "a scale, a musical scale," from Greek diagramma "geometric figure, that which is marked out by lines," from diagraphein "mark out by lines, delineate," from dia "across, through" (see dia-) + graphein "write, mark, draw" (see -graphy). Related: Diagrammatic; diagrammatically. The verb, "to draw or put in the form of a diagram," is by 1822, from the noun. Related: Diagrammed; diagramming.
The concept used to settle this problem is based on molecular orbit diagram. You are watching: Construct the molecular orbital diagram for h2 and then identify the bond order. A molecular orbital diagram is used to define chemical bonding in a molecule. This chart is based ~ above the molecular orbital theory.
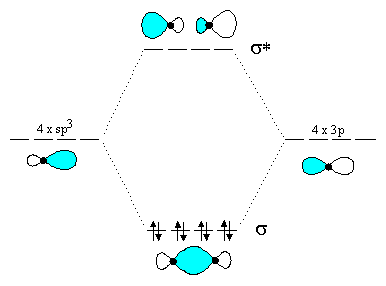
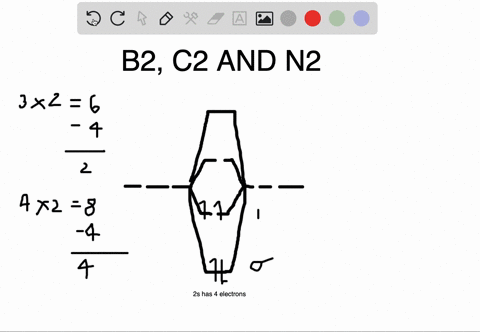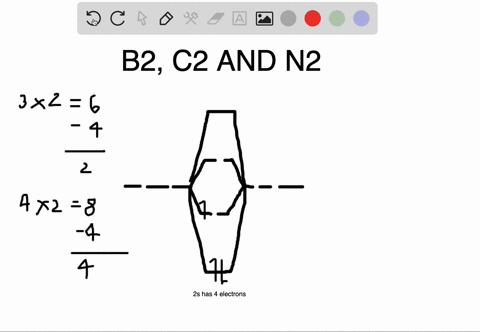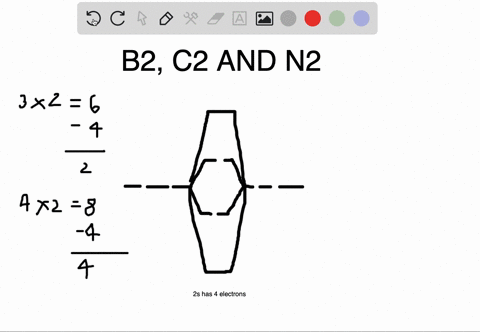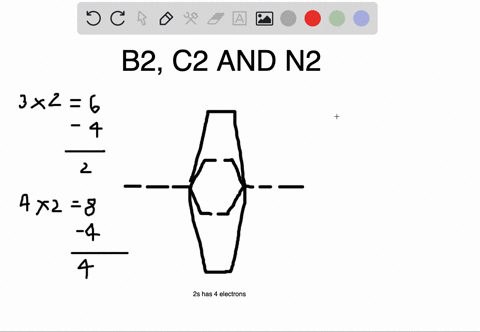
Molecular orbital diagram for b2. B2 molecular orbital diagram. This also causes a large jump in energy in the 2p σ orbital. For example when two hydrogen atoms bond a σ1s bonding molecular orbital is for med as well as a σ1s antibonding molecular orbital. Valence bond model vs. The molecular orbital diagram for an o 2 molecule would there ...
A molecular orbital model for 1,3-butadiene is shown below. Note that the lobes of the four p-orbital components in each pi-orbital are colored differently and carry a plus or minus sign. This distinction refers to different phases, defined by the mathematical wave equations for such orbitals.
I am a graduate student in Chemistry in an Inorganic class. We have touched on Tanabe-Sugano diagrams in class, but did not go into deep explanation. The homework problems I have been given use these diagrams, but I am not sure how to use them. What I understand: These diagrams show the allowed transitions for both high and low spin complexes. I thought that term symbols were used to read these diagrams, but the E, T, and A designations look more like molecular orbital labels. Somehow, the de...
Various methods (Hartree-Fock methods, semi-empirical methods, Density Functional Theory, Molecular Mechanics) used to optimize a molecule structure feature the same basic approach but differ in the mathematical approximations used. The geometry optimization procedure calculates the energy at an initial geometry of a molecule and then proceeds to search a new geometry with a lower energy ...
As stated previously, continuous bands of energy are formed due to the combinations of molecular orbitals close in energy. Of course, due to the mass amounts of different molecular orbital mixings, bands of varying energy will form. The difference between these band energies is known as the band gap, as indicated in Figure 2. Figure 2.
Molecular orbitals provide a great model for demonstrating molecule bonding via molecular orbital theory. Types of Molecular Orbitals. According to molecular orbital theory, some types of molecular orbitals are formed by the linear combination of atomic orbitals. These orbitals are described in more detail below.
also sub-orbital, 1803 of the eye; 1959 of a planet, from sub- + orbital (adj.). Related: Suborbitally.
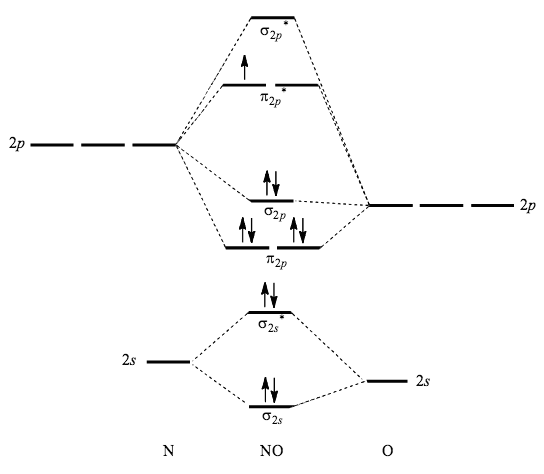
(c) Their molecular orbital diagrams are more symmetrical than those of homonuclear diatomic molecules. (d) The bonding molecular orbitals have more of the ...
Hope the information shed above regarding NCERT MCQ Questions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure with Answers Pdf free download has been useful to an extent. If you have any other queries of CBSE Class 11 Chemistry Chemical Bonding and Molecular Structure MCQs Multiple Choice Questions with Answers, feel ...
\[[https://pubchem.ncbi.nlm.nih.gov/periodic-table/png/Periodic\_Table\_of\_Elements\_w\_Chemical\_Group\_Block\_PubChem.png](https://pubchem.ncbi.nlm.nih.gov/periodic-table/png/Periodic_Table_of_Elements_w_Chemical_Group_Block_PubChem.png) \] or \[[https://ptable.com/#Properties](https://ptable.com/#Properties) \] In the last post, I mentioned the concept of activation barriers: reactions require an energy input to proceed from starting materials to a transition state even if there is a net re...
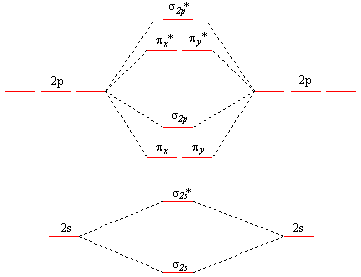
Jan 25, 2020 — What we see here is a molecular orbital interaction diagram. The middle of the diagram is just the molecular orbital energy diagram.
As a science communicator, I'm very interested in raising the science literacy of the general public. That's why I've created a series of general chemistry tutorials that cover the topics in a year of high school chemistry, but in as clear and concise a way as possible. There are quantitative practice problems for those who want to learn the math, but it's also for any person to simply watch in a relaxed way in order to absorb the content and expand their understanding. I genuinely believe it's ...
Quantum-chemical calculation of most important parameters of molecular and electronic structures of octa-carbon C8 having cubic form (bond lengths, bond and torsion angles) using CCSD(T)/QZVP and DFT B3PW91/QZVP methods, has been carried out. NBO analysis data and HOMO/LUMO images for this compound are presented, too. Good agreement was found between the structural data obtained using the ...
VSEPR theory (molecular shapes), bond polarity, molecular polarity and IMFs ... 213XZ948 Felicia Mo AP Chemistry Unit 2,3 Question #1 1a) 1b) C2H2 ... There are 60 multiple-choice questions and 7 free- response questions.. orbitals (called molecular orbitals) spread over entire molecule.
1. Draw a molecular orbital diagram and determine the bond order expected for the molecule B2. For full credit on MO diagrams,. • label increasing ...
Feb 16, 2017 · How To Draw The Molecular Orbitals of The Allyl Cation, Allyl Radical And Allyl Anion. Drawing the molecular orbitals of a pi system like allyl (3 conjugated p-orbitals) is a bit like construction: build the house (orbitals) first, and fill it with people (electrons) second.
In book: BOND ORDER OF DIATOMIC SPECIES WITHOUT MOLECULAR ORBITAL THEORY (MOT) (pp.44-54) Publisher: WikiEducator, Open Educational Resource (OER) Foundation, Otago Polytechnic, Dunedin, New ...
Molecular Orbital theory is a concept of quantum mechanics that attempts to explain the chemical bonding inside any molecule. In this theory, we get to know that valence electrons can be shared amongst all constituent atoms and atomic orbitals from different atoms combine to form molecular orbitals ( MOs ).
Cross posted from chem help so forgive me if for some reason you’re seeing this twice now. I’m working through a problem set for one of my classes and so far it’s had me make the SALCs and molecular orbital diagram for NH3. Now it’s asking me to go from constructing the MO diagram to identifying the ground electronic state of the molecule. I’m guessing what I need is some kind of wave function to describe the population of each orbital in the ground state. But I’m not sure how to get there fro...
Sequential Easy First Hard First. Play as. Quiz Flashcard. Hybridization is the mixing of atomic orbitals into new hybrid orbitals, suitable for the pairing of electrons. Molecular Geometry highly uses this concept. The quiz below is on the subject. May seem hard, but try it out. Questions and Answers. 1.
"the property of certain compounds by virtue of which they differ in molecular weight and chemical properties though formed from the same elements in the same proportion," 1866, from polymer + -ization.
Q. Draw a picture that shows all three 2p orbitals on one atom and all three 2p orbitals on another atom. (c) How many antibonding orbitals, and of wh... Solved • Oct 5, 2021. Molecular Orbital Theory. Q. For each of these contour representations of molecular orbitals, identify (d) the locations of nodal planes.
SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. SF4 or sulfur tetrafluoride is a compound that has a distinct odor of sulfur or rotten eggs. This compound is generally identified as being a colorless gas. The molecular weight of this compound is calculated to be 108.6 g/mol. SF4's boiling and melting points are -38 ...
also intraorbital, 1836, from intra- "within" + orbit (n.) + -al (1).
Molecular orbitals are of two types - bonding and antibonding. The two types of bonds are σ - bond and π − bond. The s -orbitals of one atom can overlap with the s, p, d, f, orbital of another atom such that the overlapped region is symmetrical about the internuclear axis. Similar symmetrical overlaps are also possible among p, d and f ...
1868, from normal (in reference to molecular structure) + epinephrine.
The filled molecular orbital diagram shows the number of electrons in both bonding ... You can practice labeling and filling molecular orbitals with this ...
Hello, I'm a chemistry student with a personal interest in programming and I have, for my personal project, tried to make a crude program for calculations using the Hückel theory of molecular orbitals. From the mathematical standpoint, the problem boils down to filling up a giant matrix with x's on the main diagonal and up to three 1's in every row/column. Then you have to take the determinant of the said matrix and solve the generated polynomial. The solutions are then displayed both as a list ...
Hi /r/Chempros. Have you ever shed blood and tears on writing a script, only to find after a few weeks that something really similar had already been done? Have you ever created a specific tool but didn't really had the time or the right place to share it with your colleagues? Have you ever seen a really useful reddit post that you wish you had saved? I have, and after a quick exchange with our dear mod /u/wildfyr I've decided to post this thread. #Scope I would like for it to be a location ...
While learning about ferrocene I've come across this molecular orbital diagram. What particularly confused me is the nature of the a'1g orbital. My professor taught that a'1g and e2g are considered HOMO bonding orbitals, but this MO diagram clearly shows the a'1g as an MO with antibonding character.
A diagram is a symbolic representation of information using visualization techniques. Diagrams have been used since prehistoric times on walls of caves, but became more prevalent during the Enlightenment. Sometimes, the technique uses a three-dimensional visualization which is then projected onto a two-dimensional surface. The word graph is sometimes used as a synonym …
A machine learning algorithm has identified an antibiotic that kills E. coli and many other disease-causing bacteria, including some strains that are resistant to all known antibiotics. To test it, mice were infected on purpose with A. baumannii and C. difficile and the antibiotic cleared the mice of both infections. "The computer model, which can screen more than a hundred million chemical compounds in a matter of days, is designed to pick out potential antibiotics that kill bacteria using dif...
Learning Goals. - visualize the change in bonding and antibonding molecular orbitals as electronegativities of bonding atoms are changed. - explain how changes in the atomic orbital contribution to molecular orbitals results in a continuum of chemical bonding (i.e. covalent, polar, and ionic bonds)
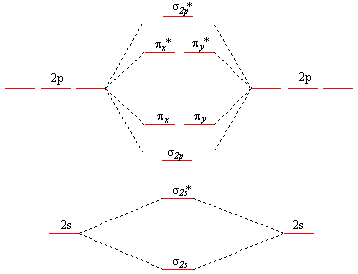
Jun 05, 2017 · General Notes on Molecular Orbital Diagrams. The Y-axis of a MO diagram represents the total energy (not potential nor Gibbs Energy) of the orbitals. Individual atomic orbitals (AO) are arranged on the far left and far right of the diagram. Overlapping atomic orbitals produce molecular orbitals located in the middle of the diagram.
I am having difficulty understanding how to understand and draw frontier molecular orbital. I was wondering if someone can help and also try to walk me through this following problem as well? http://postimg.org/image/f9i5ztu3h/ The second electrophile that should be formed is the center carbon triple bonded to the oxygen. I think that is right because that will allow me to kick off the the ALCL4 (-) group. I guess my problem is more of a visual understanding of the problem in order to see whic...
[Book 1 of The HEL Jumper](https://www.reddit.com/7oulr8) [Book 2 of The HEL Jumper](https://www.reddit.com/akws1r) \----- [Previous](https://redd.it/idztze) | [First](https://www.reddit.com/eo9svn) | [Next](https://redd.it/iqrcc1) | [Patreon](https://www.patreon.com/SabatonBabylon) Thanks to Big_Papa_Dakky, Darth_Android, bloblob, AMERICUH, The_Real_Jumper, Mr_Polygon, Krystalin, Damned_Thrice, Mamish, Vikairious, Sam_Berry, RedHawkdude, KillTech, LilLaussa, Daddy_Talon, Gruecifer, Gaelan_D...
Quantum Mechanics does not predict Atomic and Molecular Orbitals. Michele Cini. Sep 12 · 13 min read. Theoretical atomic, molecular and condensed-matter physicists and also theoretical chemists make proper use of orbitals and related techniques. They are aware that such concepts, while useful, can be potentially misleading if taken naively.
"pertaining to schemes," 1701, from Latin stem of scheme (n.) + -ic. Noun meaning "diagram" is first attested 1929. Related: Schematical (1670s).
Hi folks, I'm trying to create some visualizations for advanced high school/introductory college chemistry education. In particular, I'd like to run some computational chemistry models to produce molecular orbital representations and MO diagrams of some simple molecules and reactions. But as I've been running some test models and compiling my ideas, I've had some questions come up that I'm hoping you could help answer. First, does anyone have recommendations for specific (free) programs to use...
CHEM1611 Worksheet 5: Introduction to Carbon Chemistry. Model 1: Bonding in Organic Molecules ... to produce hybrid orbitals, is known as hybridization.. a) On the structure above, identify the hybridization state of all carbon atoms. b) Draw a picture below that clearly shows the interacting orbitals of all ....




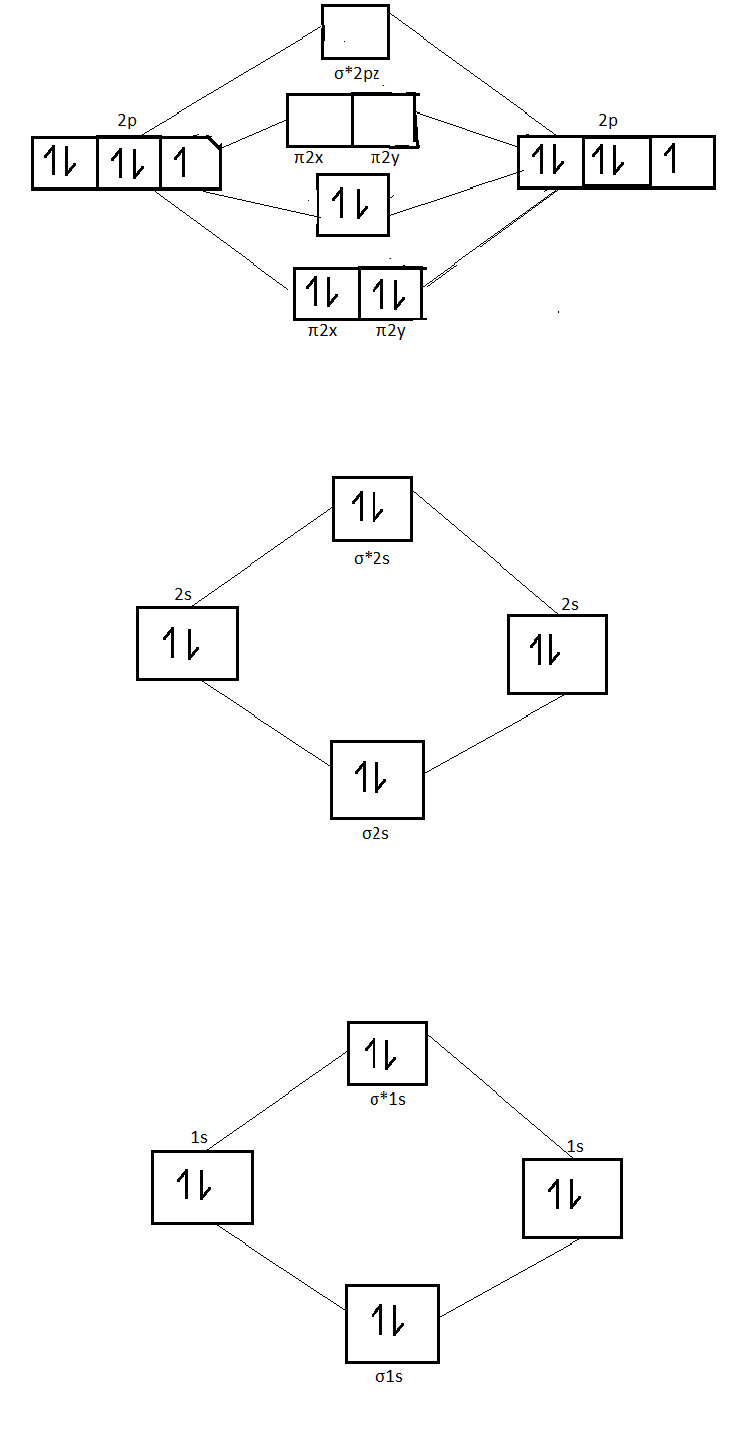


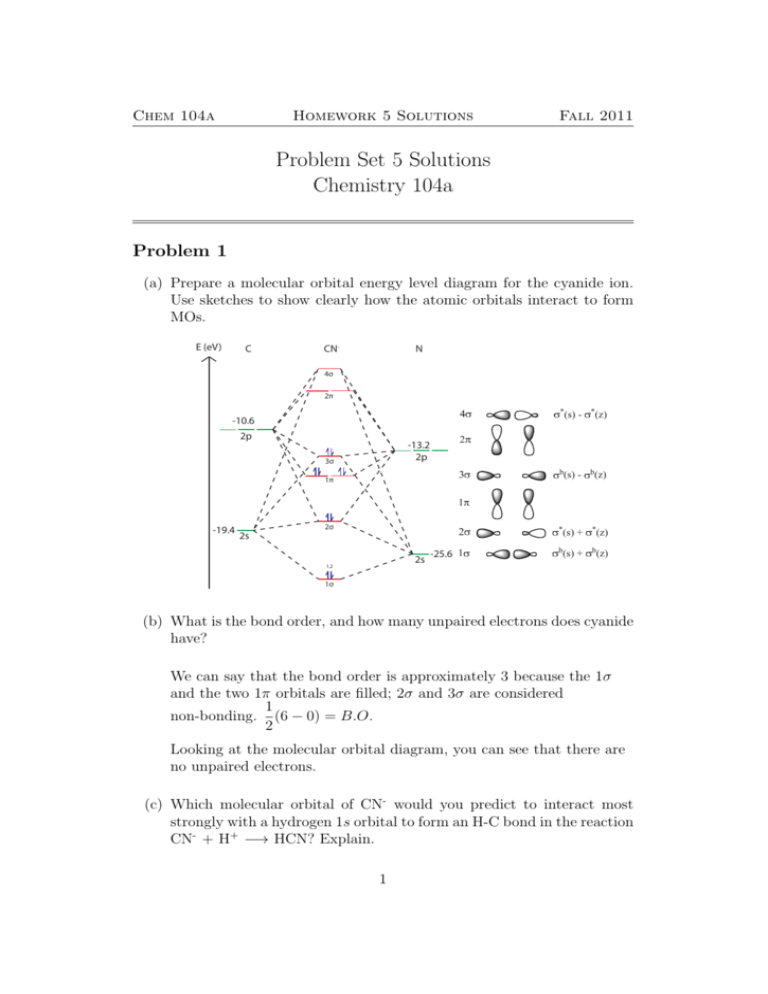
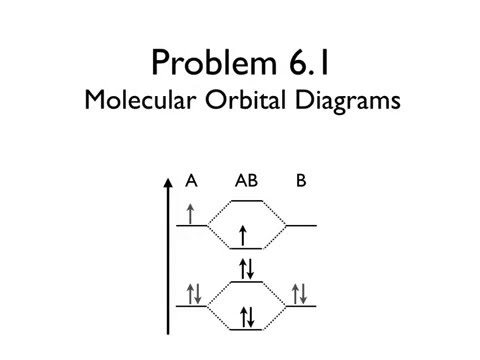
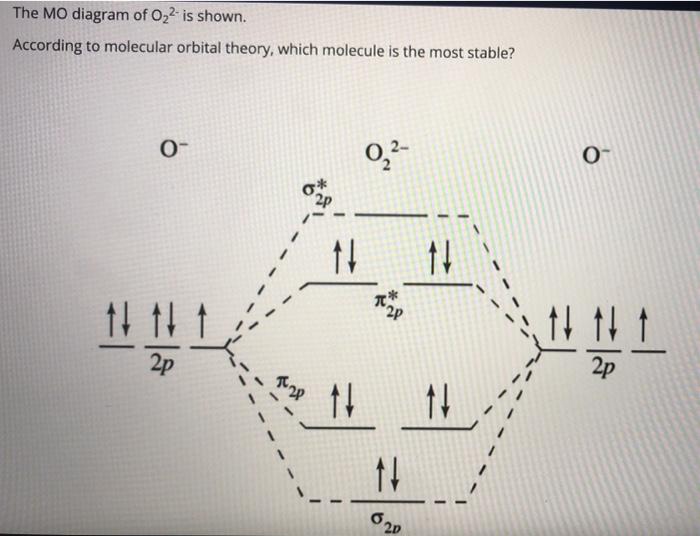











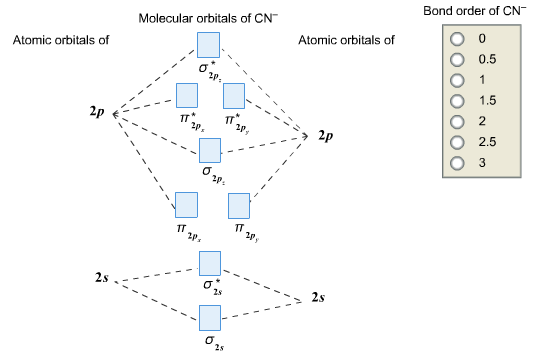

0 Response to "41 molecular orbital diagram problems"
Post a Comment