44 electron dot diagram of sulfur
Ans: Electron dot structure of an atom is basically a representation of the electrons (valence electrons) in the outermost shell… View the full answer Transcribed image text : 19) Which is the electron dot structure of sulfur? 1:31Because the Sulfide ion (S2-) has an extra electron (the negative sign denotes an extra electron) we need to ...24 Aug 2018 · Uploaded by Wayne Breslyn
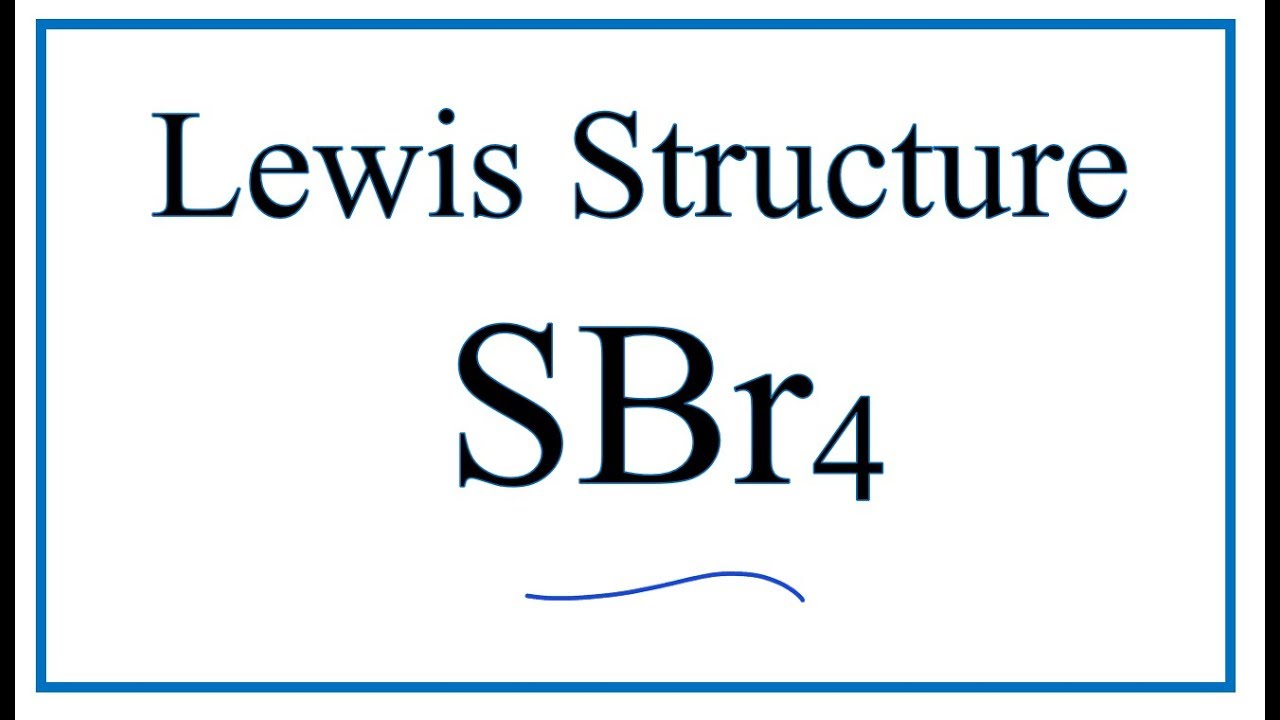
1:29A step-by-step explanation of how to draw the SBr2 Lewis Dot Structure (Sulfur dibromide).For the SBr2 ...11 May 2013 · Uploaded by Wayne Breslyn

Electron dot diagram of sulfur
Best Answer. Copy. The electron dot diagram for a lone uncharged Sulfur particle is an S with 6 electrons arranged around it (2 orbitals with 2 electrons and 2 orbitals with 1). Wiki User. A step-by-step explanation of how to draw the Lewis dot structure for S (Sulfur). I show you where Sulfur is on the periodic table and how to determine how ... 1:38Once we know how many valence electrons there are in SI2 we can distribute them around the central atom ...8 Oct 2019 · Uploaded by Wayne Breslyn
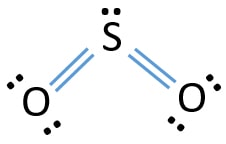
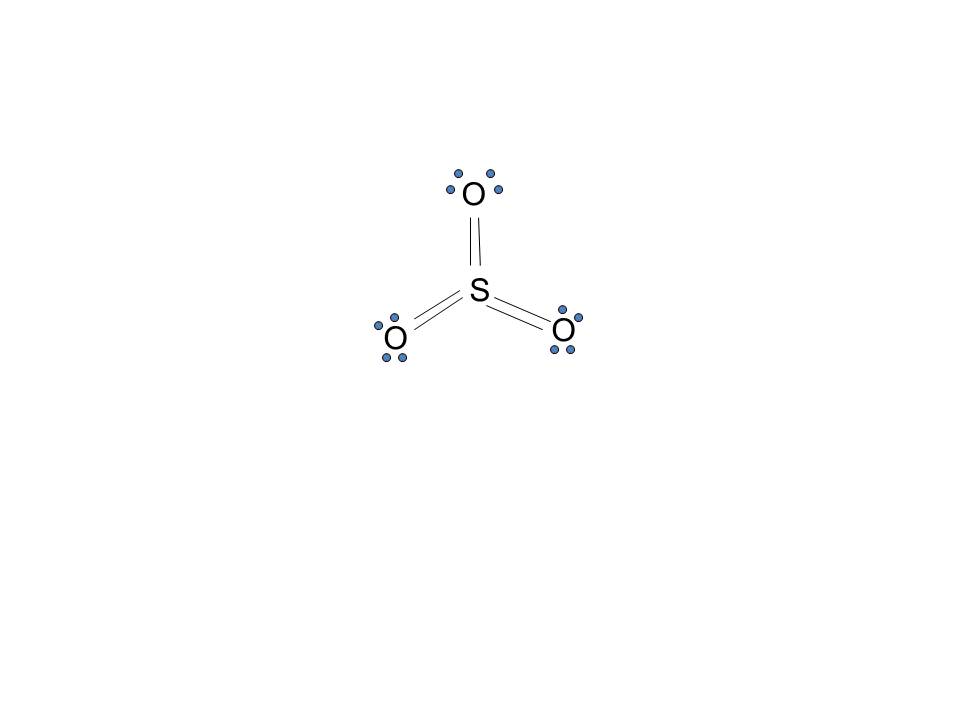
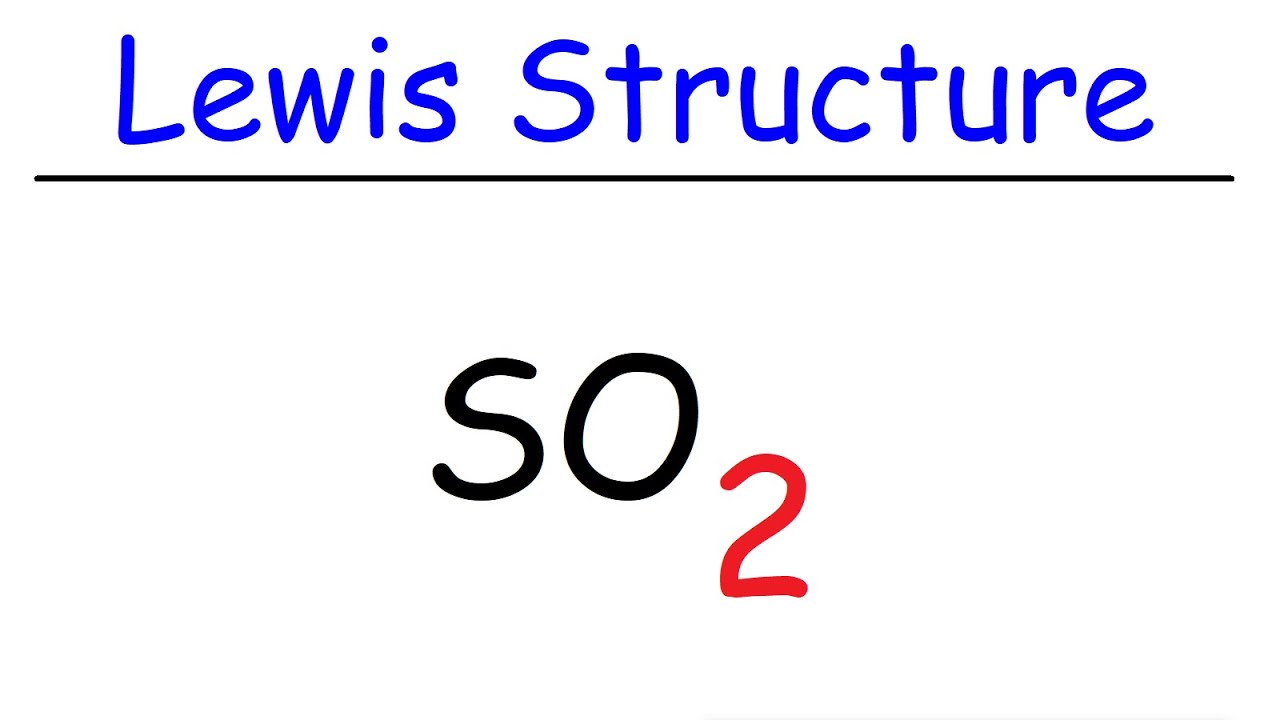
Electron dot diagram of sulfur. Here is the answer for the question - Draw the electron-dot formula for the element sulfur.. You'll find the correct answer below Draw the electron-dot formula for the element sulfur. The Correct Answer is. Sulfur belongs to group 16 of the periodic table, so it has six valence electrons. The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access . Drawing the Lewis Structure for SO 2. 23 Mar 2020 — Note: Sulfur is in Group 16 (sometimes called Group VI or 6A). Since it is in Group 6 it will have 6 valence electrons. 1:18A step-by-step explanation of how to draw the SCl4 Lewis Dot Structure (Sulfur Tetrachloride).For the SCl4 ...28 Jun 2013 · Uploaded by Wayne Breslyn
Sulfur dibromide has a dot structure that begins with the S atom in the center. On the left and right sides are a singly bonded Br atom. On the unbonded sides of each atom is a single pair of dots. IMPORTANT: no Lewis diagram is complete without formal charges. Lewis diagrams are drawn to examine mechanisms so knowing which parts of a molecule are electron defficient (+) and which are electron rich (-) is vital. It is best to have a formal charge of … A neutral sulfur atom has 6 electrons regardless of the isotope. Lewis dot diagram for sulfur. The key is to understand the steps and practice. The lewis structure for so 2 requires you to place more than 8 valence electrons on sulfur s. You might think youve got the correct lewis structure for so 2 at first. Drawing the lewis structure for so 2. Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). Lewis dot structure will have 4 paired dots around Sulfur atom.
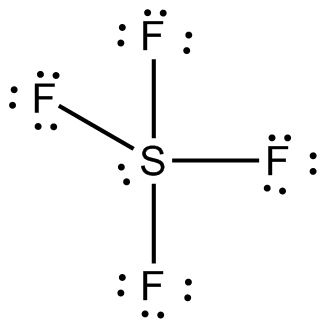
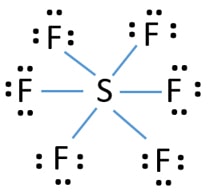
Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another (pairs of dots can be used instead of lines). Excess electrons that form lone pairs ... What would be the electron dot structure of a molecule of Sulphur which is made up of eight atoms of Sulphur? (Hint – The eight atoms of Sulphur are joined together in the form of a ring). Answer: Sulphur has an atomic number of 16 and an electronic configuration of 2,8,6. The valance electrons on the sulphur atom are six. Answer. 29. What is the correct electron dot diagram for a neutral atom of SULFUR ? Answer : Surfum (s) electronic configuration, is? 252 286 352 3p 4 valence shell for Sulfur 352 384 The ne fone, electron dot diagram of a neutral sulfur is. :5: 01.01.2014 · WARNING. This is a LONG document. It covers all possible shapes for molecules with up to six electron pairs around the central atom. STEPS INVOLVED There are three basic steps to determining the molecular shape of a molecule: Write the Lewis dot structure of the molecule. That gives you the steric number (SN) — the number of bond pairs and lone pairs around the central atom.
27.11.2019 · Wang, H. C. et al. Cadmium-free InP/ZnSeS/ZnS heterostructure-based quantum dot light-emitting diodes with a ZnMgO electron transport layer and a …
1:08A step-by-step explanation of how to draw the SF2 Lewis Dot Structure (Sulfur difluoride).For the SF2 ...25 May 2013 · Uploaded by Wayne Breslyn
For example, the Lewis electron dot diagram for calcium is simply ... of anions from atoms, as shown below for chlorine and sulfur: Two diagrams are shown.
1:38Once we know how many valence electrons there are in SI2 we can distribute them around the central atom ...8 Oct 2019 · Uploaded by Wayne Breslyn
A step-by-step explanation of how to draw the Lewis dot structure for S (Sulfur). I show you where Sulfur is on the periodic table and how to determine how ...
Best Answer. Copy. The electron dot diagram for a lone uncharged Sulfur particle is an S with 6 electrons arranged around it (2 orbitals with 2 electrons and 2 orbitals with 1). Wiki User.
A State The Electron Dot Structure For Calcium And Sulphur B Show The Formation Of Cas By The Transfer Of Electrons Sarthaks Econnect Largest Online Education Community

Draw The Lewis Dot Structure For So32 Determine The Electron Geometry And Molecular Shape Of This Molecule Study Com
Sulfur Dioxide Lewis Structure Molecule Molecular Geometry Resonance Silicon Dioxide Structure Angle Text Chemistry Png Pngwing

Draw The Lewis Structure For The Sulfur Trioxide So 3 Molecule Be Sure To Include All Resonance Structures That Satisfy The Octet Rule Study Com

Tro Ic How Many Dots Showing Electrons Are Around The S In The Lewis Structure Of A Sulfur Atom Ppt Download






















0 Response to "44 electron dot diagram of sulfur"
Post a Comment