44 potassium electron dot diagram
How to draw KBr Lewis Structure? - Science Education and ... The first step is to put seven valence electrons around the potassium atom as given in the figure. Step-2: Lewis Structure of KBr for counting valence electrons around the terminal potassium atoms As a result, bromine is the third atom in the periodic table's halogen family group. Potassium is the first member of the alkaline metal family. Lewis Dot Structures Worksheet - Mr. Walsh's Class Draw the Lewis Dot Structure. Notes: Scientists use. Lewis Dot Structures. to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons.
potassium lewis dot structure - Creed On Lake lewis dot structure potassium nitrate is a solid at room temperature and appears as a odorless white solid it has a molar mass of 101.1032 g/mol and a density of 2.109 g/cm3 it has a melting point of 334 °c and a boiling point of 400 °c after hydrogen fluoride, kf is the primary source of the fluoride ion for applications in manufacturing and in …

Potassium electron dot diagram
38 which lewis electron-dot diagram represents the bonding ... Which lewis electron-dot diagram represents the bonding in potassium iodide. Lewis Dot Diagram Iodine When we write the . Lewis dot structures help predict molecular geometry. Place the remaining four electrons around the iodine atom to complete the structure. Electron Dot Structures - Helpful tools in thinking about bonding. N2 Lewis Structure, Molecular Geometry, and Hybridization ... Mar 30, 2022 · An electron that is placed in the outermost shell of an atom is known as a valence electron. To determine the number of valence electrons, you can simply note down the Group number of the element from the Periodic Table. Lewis used lines to state a covalent bond between two electrons and each electron is denoted by a dot in the diagram. Sulfur Lewis Dot Structure:Drawing,Several Compounds And ... Potassium and Sulfur lewis dot structure. Potassium is a 'group 1' element in periodic table. It has one outer most shell electron in 4s electronic shell ([Ar] 4s1). Potassium atom transfers this outer most shell electron to maintain Octet rule and become a positive ion. The electron is accepted by Sulfur atom and form Sulfide ion.
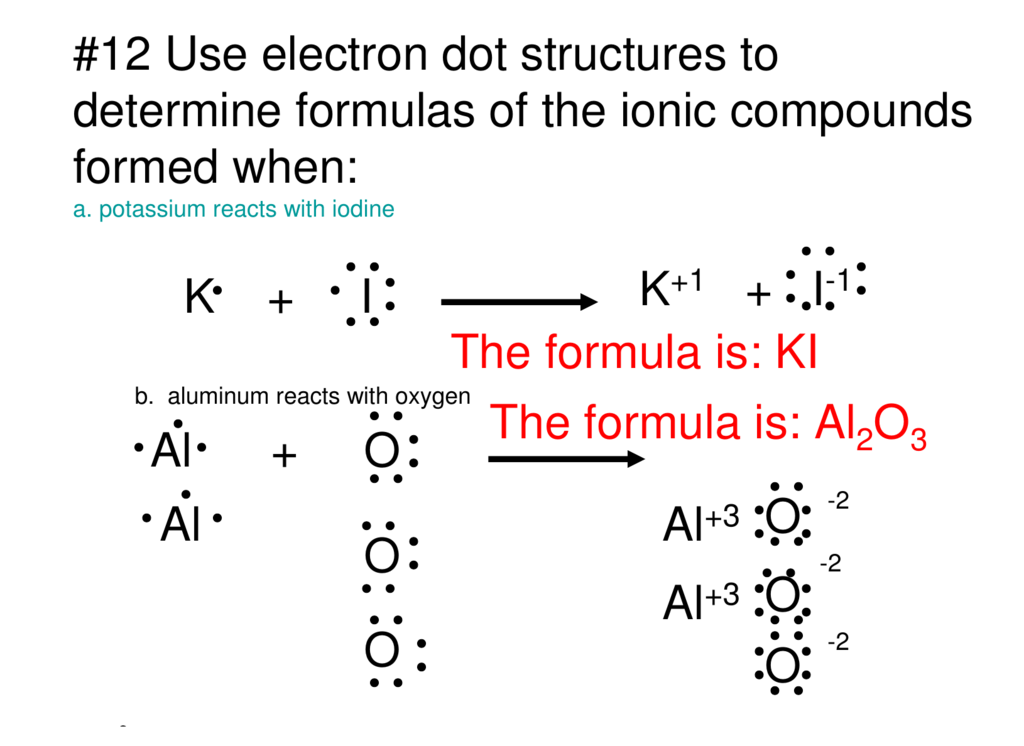
Potassium electron dot diagram. How to Draw the Lewis Dot Structure for KNO3 (Potassium ... A step-by-step explanation of how to draw the KNO3 Lewis Dot Structure.For KNO3 we have an ionic compound and we need to take that into account when we draw ... Which Lewis dot structure represents bonding in potassium ... In this case, option 1 is the correct representation for potassium iodide using dot electron structure because potassium atom transfers its one valence electron to iodine forming the iodide ion having eight valence electrons as shown. This gives iodine a negative charge and potassium a positive charge as shown in the structure in option 1. NO3 Lewis Structure, Molecular Geometry, and Hybridization 1 day ago · In 1916, American chemist, Gilbert N. Lewis introduced the concept of electron dot structure. Below are some rules to frame any compound’s Lewis dot structure. 1. Follow the octet rule where an atom should complete its outermost shell by the total number of 8 electrons. (Exceptions are hydrogen and boron elements) 2. write the electron dot structure for Potassium Chloride ... The correct answer for ions present in KCl is K⁺ and Cl⁻ which forms KCl by ionic bond (complete transfer of electron from K to Cl ) . ... Since Potassium has 1 electron in its outermost shell , so its electron dot structure involves K with one dot . Explanation: see the pics uploaded here!! Advertisement Previous Next
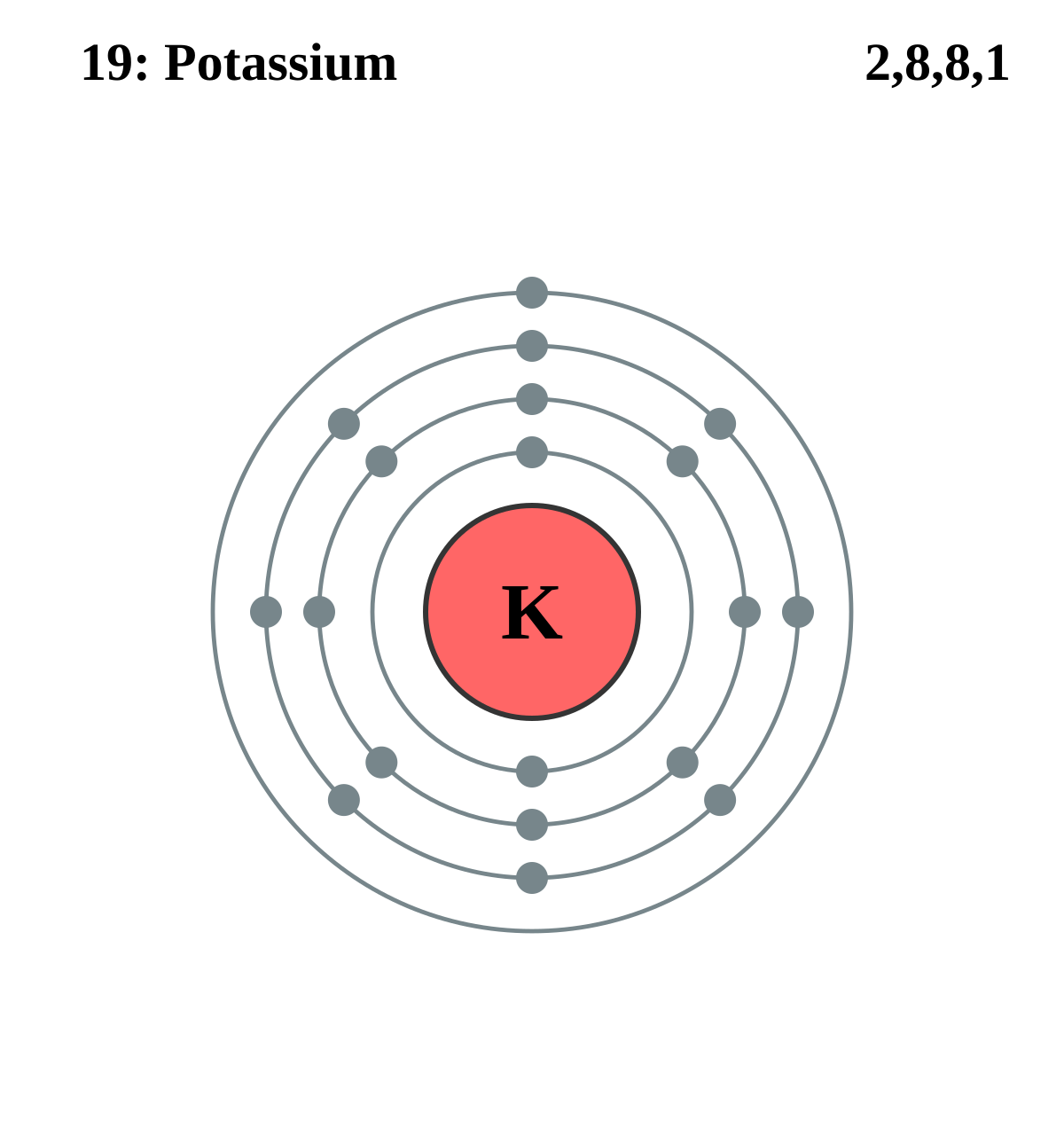
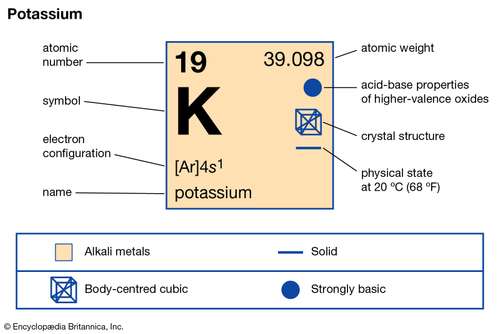
The Lewis Dot Structure for KCl - MakeTheBrainHappy Please find above the Lewis Dot Structure for KCl (Potassium Chloride). As per usual you could replace the one bond with two electrons. In the case for KCl the electronegativity difference between potassium and chloride is so strong (.82 vs. 3.16, respectively) that the bond is considered ionic. Potassium Bohr Model - How to draw Bohr diagram for ... Electron dot diagram of a Potassium atom Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Potassium, we got to know, it has only 1 valence electron. So, just represent the 1 valence electron around the Potassium atom as a dot. The electron configuration of Potassium How do you draw electron dot diagrams ... How to Draw a Lewis Dot Structure. Determine the total number of valence electrons to be depicted in the Lewis diagram. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Determine how many electrons must be added to central element. Conejo Valley Unified School District > Homepage Oct 26, 2017 · Draw Lewis dot diagrams of the following atoms. 2. Carbon 3. Neon 1. Calcium 4. Hydrogen Ionic bonding occurs when a metal transfers one or more electrons to a nonmetal in an effort to attain a stable octet of electrons. For example, a Lewis dot diagram can depict the transfer of an electron from otassium to chlorine.
Lewis Dot Structure for Potassium - YouTube A step-by-step explanation of how to draw the Lewis dot structure for K (Potassium). I show you where Potassium is on the periodic table and how to determine... PDF Lewis electron dot diagram for potassium iodide Electron diagram for potassium. No one will be able to draw a diagram here like this is a reply only text. 3 Composed 1 is ionic and composed 2 is molecular. Place the remaining four electrons around the iodine atom to complete the structure. CN- lewis structure, molecular orbital diagram, and, bond order Also, using the Molecular orbital diagram of CN-we can also find its bond order which helps us to predict its bond length and stability as well. Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2. Electron Configuration for Potassium (K) In writing the electron configuration for Potassium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Potassium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
potassium fluoride lewis dot structure - ofcs.org A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Li+ OR. boron properties uses amp facts britannica com. What is the Lewis electron dot structure for potassium and.
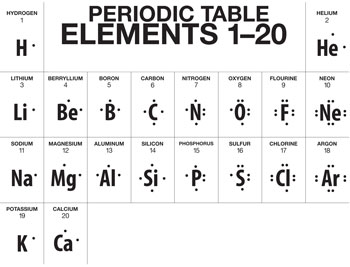
lewis dot diagrams.pdf LEWIS DOT DIAGRAMS Name Lewis diagrams are a way to Indicate the number of valence electrons aroun an atom. A\5Ö are all examples of this type of diagram. Draw Lewis dot diagrams of the following . l. calcium 2. potassiürn rgon 4. aluminum 2-3 -B 5, bromine 6. carbon . 7. helium 8. oxygen 9. phosphorus . hy rogen
What is the electron dot diagram for potassium? - Answers What is the electron dot notation for potassium? K* (star means the dot) An electron dot diagram uses the symbol of an element and dots to represent the? In an electron dot diagram, the dots...
LEWIS DOT DIAGRAM - A Science Class Project Since potassium is in column one it has only 1 valence electron. Creating the diagram: The center of any Lewis Dot Diagram must be the element symbol. In this case it would be "K" for potassium. The electrons have to be placed in a certain order around the element symbol. They are placed in pairs on each side of the element symbol.
Oxygen Bohr Model - How to draw Bohr diagram for Oxygen(O) atom Electron dot diagram of the Oxygen atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Oxygen, we got to know, it has 6 valence electrons. So, just represent these 6 valence electrons around the Oxygen atom as a dot.
Why do potassium and sodium have the same number of dot ... Why do sodium and potassium have the same number of dots in their electron dot diagrams? Electron dot diagrams show the number of valence electrons and whether they are paired or unpaired. One of...
Draw the Lewis dot diagram for potassium. | Study.com Draw the Lewis dot diagram for potassium. Lewis Electron Dot Diagram: Lewis electron dot diagram is a diagram which shows the number of valence electron (s) that an atom has. The valence electrons...
How would you represent potassium and bromine using an ... Potassium's electron configuration looks like this ["K"]: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^1 As you can see, potassium has one valence electron located on the fourth energy level in the 4s-orbital. This means that its electron dot diagram will feature its chemical symbol and one dot, usually placed above the symbol.
Lewis Electron Dot Diagrams - Welcome to CK-12 Foundation Since the Lewis electron dot diagrams are based on the number of valence electrons, it would hold true that the elements in the same group would have the same electron dot diagram. In other words, if every element in Group 1A has valence electron, then every Lewis electron dot diagram would have one single dot in their Lewis electron dot diagram.
Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
%tiNitrogen Lewis Dot Structure:Drawing,Several Compounds ... Potassium and Nitrogen lewis dot structure. Potassium is a 'group 1' element in periodic table. It has one outer most shell electron in 4s electronic shell. Potassium donates this valence electron to satisfy Octet rule and become a positive ion. The electron is accepted by Nitrogen atom and form nitride ion.
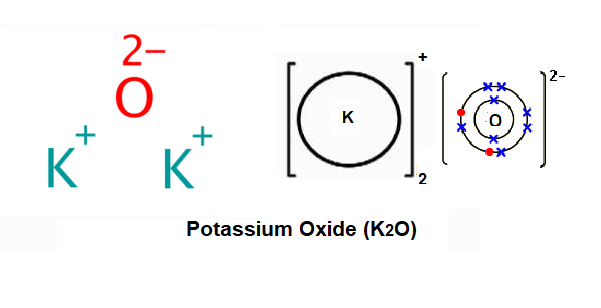
Potassium Oxide - Structure, Properties, and Uses of K2O Potassium oxide is an ionic compound formed by combining potassium and oxygen. It carries the chemical formula K2O. Potassium cannot be found free because it is too reactive. It has valency +1 and combines readily with oxygen atoms forming K 2 O. The oxide, K 2 O, is obtained as a grey crystalline substance when potassium is oxidized; potassium ...
How to draw KI Lewis Structure? - Science Education and ... The first step is to put seven valence electrons around the potassium atom as given in the figure. Step-2: Lewis Structure of KI for counting valence electrons around the terminal potassium atoms As a result, iodine is the fourth atom in the periodic table's halogen family group. Potassium is the fourth member of the alkaline metal family.
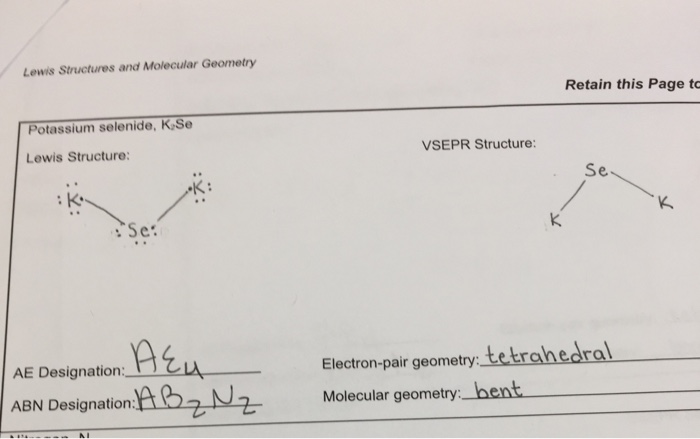
Draw electron dot representation for the formation of ... The electron dot structure for the formation of potassium chloride is given above. Solve any question of Chemical Bonding and Molecular Structure with:-.
potassium fluoride lewis dot structure - ICC A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The bifluoride on heating yields potassium fluoride: Platinum or heat resistant plastic containers are often used for these operations.
Draw electron dot representation for the formation of ... >> Draw electron dot representation for the Question 10 fa) Write the electron-dot structures for potassium and chlorine. 16 Show the formation of KCl by the transfer of electrons. Name the ions present in this compound, KCI. (Atomic number of K=19, CI = 17) Solution Verified by Toppr
Lewis Structure Questions and Answers | Study.com Draw an electron dot diagram for beryllium, showing the correct number of valence electrons. ... Draw the Lewis dot structure of potassium and answer the following questions. a. What is its ...
Sulfur Lewis Dot Structure:Drawing,Several Compounds And ... Potassium and Sulfur lewis dot structure. Potassium is a 'group 1' element in periodic table. It has one outer most shell electron in 4s electronic shell ([Ar] 4s1). Potassium atom transfers this outer most shell electron to maintain Octet rule and become a positive ion. The electron is accepted by Sulfur atom and form Sulfide ion.
N2 Lewis Structure, Molecular Geometry, and Hybridization ... Mar 30, 2022 · An electron that is placed in the outermost shell of an atom is known as a valence electron. To determine the number of valence electrons, you can simply note down the Group number of the element from the Periodic Table. Lewis used lines to state a covalent bond between two electrons and each electron is denoted by a dot in the diagram.
38 which lewis electron-dot diagram represents the bonding ... Which lewis electron-dot diagram represents the bonding in potassium iodide. Lewis Dot Diagram Iodine When we write the . Lewis dot structures help predict molecular geometry. Place the remaining four electrons around the iodine atom to complete the structure. Electron Dot Structures - Helpful tools in thinking about bonding.




![Potassium carbonate]](https://www.degruyter.com/document/doi/00.0000/IUPAC.iupac.compound.11430/asset/images/11430.png)










![Expert Answer] Write the electron dot structure for potassium ...](https://us-static.z-dn.net/files/dae/42ea31befae64e0549ebc6aa19bd29da.png)



![Answered] Add electron dots and charges as necessary to show ...](https://us-static.z-dn.net/files/d0f/714022937d771ff96cc1c3d4c7b44dcd.jpg)










0 Response to "44 potassium electron dot diagram"
Post a Comment