45 no+ molecular orbital diagram
How To Draw Molecular Orbital Diagram Of Co - Drawing ... Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The bonding mos are the 2σ, 1πx, 1πy, and 3σ, which gives 2 +2 +2 +2 = 8 bonding electrons. The content is presented using short focussed and interactive screencast. In molecules, electrons are present in the new orbitals called molecular orbitals. Draw the molecular energy level diagrams of NO+, NO, and ... Bond order can be calculated by the difference between the bonding electrons and non-bonding electrons in the molecular orbitals. For example, O=O has a bond ...1 answer · Top answer: Bond order of NO++ The atomic number of N is 7 and the atomic number of O is 8. There are five valence electrons present in nitrogen and...
Answer on the question #54656 – Chemistry - Assignment ... Answer: The molecular orbital diagram for CO and NO+ molecule and ion are: The bond order is the difference between the number of the bonding electrons ...1 page

No+ molecular orbital diagram
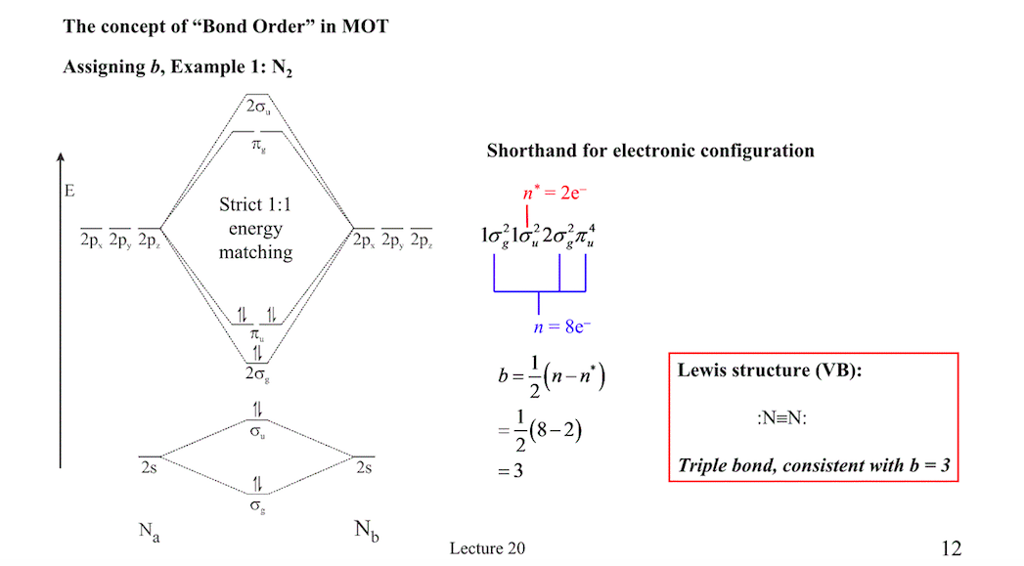
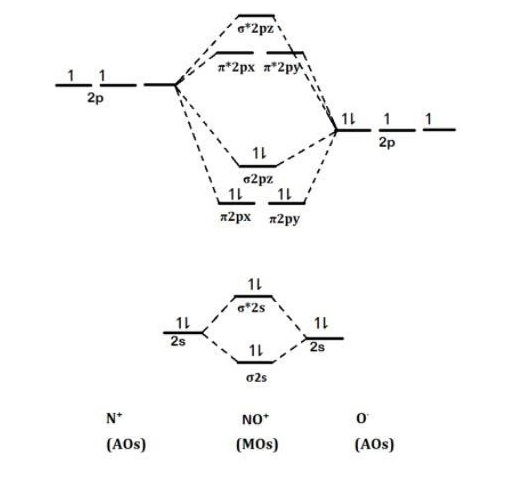
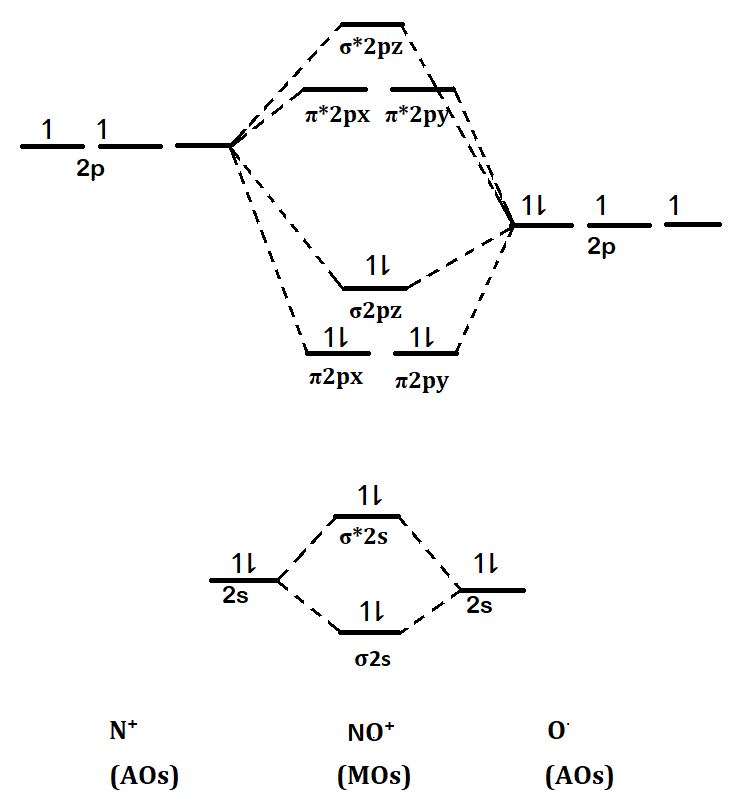
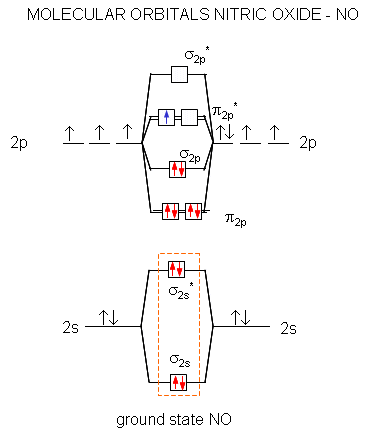
MOT(VI)NO, NO+&NO- - YouTube ¤ Molecular Orbital diagram of BN, CN, CN- ¤Molecular Orbital diagram of COhttps://youtu.be/8mufOTgvagU¤ S-P mixing OF ORBITALS ... According to the molecular orbital energy-level diagram ... The untrue statement among the options is that only one of the species is paramagnetic.. In the molecular orbital model, electrons could be filled into molecular orbitals according to the Aufbau principle in the same way electrons can be filled into atomic orbitals.. The molecular orbital configuration of the species listed are as follows;. NO = (σ2s)2 (σ⋆2s)2 (π2px)2 (π2pz)2 (σ2pz)2 ... a = Number of electrons in bonding molecular orbitals. b = Number of electrons in antibonding molecular orbitals. (1) NO+ Total number of electrons in NO + = 14 Molecular configuration can be written as (σ1s) 2 (σ*1s) 2 (σ2s) 2 (σ*2s) 2 (π) 4 (2p z) 2 a = 10 b= 4 Bond order = 1/2 (10 - 4) Bond order = 1/2 (6) Bond order = 3 Bond order of NO+ is 3.
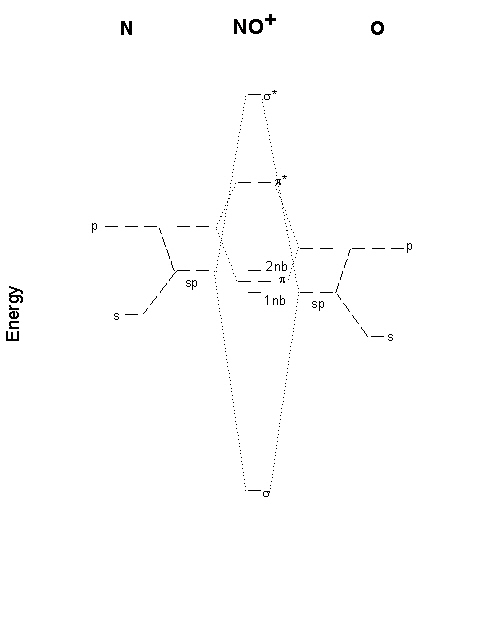
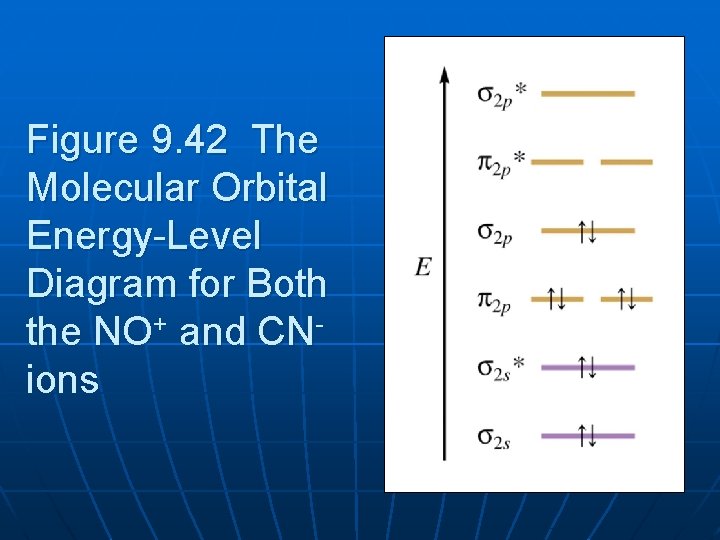
No+ molecular orbital diagram. What is the molecular orbital diagram for NO? - Quora Answer (1 of 2): This image shows the molecular orbitals of nitric oxide and the types of bonds present. Molecular Orbital Diagram For Ne2 theory, we will formalize a definition of bond order--the number of bonds between atoms in a molecule. molecular orbital energy-level diagram for the NO molecule. We assume that orbital order is the same as that for N2. The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in Molecular Orbital Diagram Of NO,NO+ And HCl Molecules ... Lecture On Molecular Orbital Diagram Of NO,NO+ And HCl Molecules Which Is Explained In Details..Molecular Orbital Energy Level Diagramhttps://youtu.be/Go6VyX...
Write the molecular orbital configurations of NO+, NO and ... Find an answer to your question Write the molecular orbital configurations of NO+, NO and NO-. Calculate their bond orders and predict which of them will be … gayatriyadav272001 gayatriyadav272001 28.05.2019 Chemistry Secondary School answered Bef2 Molecular Orbital Diagram - Wiring Diagrams Determine whether the following molecular orbitals are bonding or antibonding. ( c. 8 pts.) An incomplete MO diagram for NO+ is provided. a. (6 pts.) The point group for BeF2 is D∞h, but when determining the symmetry of the group orbitals .The linear combination of atomic orbitals always gives back the same number of molecular orbitals. Molecular Orbital Diagram of NO | All About Chemistry 1. 226. Molecular Orbital Diagram of NO. TAGS. Molecular Orbital Diagram. Previous article Molecular Orbital Diagram of CO. Next article Qualitative and Quantitative Analysis |Organic Chemistry. What is the MO electron configuration of CO? - Pvillage.org Molecular Orbital diagram of Carbon monoxide molecule (CO): CO =σ1s2,σ*1s2,σ2s2,σ*2s2, σ2px2, π2py2= π2pz2. Which of Molecular Orbital has the highest energy in CO? Which molecular orbital has highest energy in CO molecule? The HOMO orbitals are the highest energy molecular orbitals occupied by electrons.
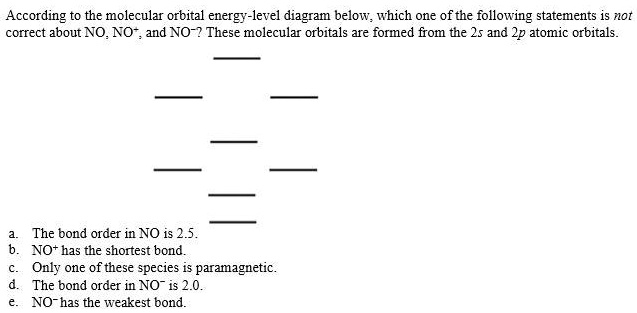
According to the molecular orbital energy-level diagram ... Answers: 3 on a question: According to the molecular orbital energy-level diagram below, which one of the following statements is not correct about NO, NO+, and NO−? These molecular orbitals are formed from the 2s and 2p atomic orbitals. By writing molecular orbital configuration for NO ... - Socratic Mar 18, 2018 — Order by bond length: NO , NO+ , NO− ... Well, the MO diagram for O2 is: ... The bond order is already calculated in the diagram.1 answer · Also see here... Bond order for NO+ Order by bond length: NO, NO+, NO− Is CO a Lewis acid? O2 is well-known to be paramagnetic, and it is one ... Doc 117 b p s xi chemistry iit jee advanced study ... - Issuu Sep 05, 2016 · Then if the basicity of acid is n, molecular weight of acid would be w2 1 × × Msalt = w1 and molecular weight of acid = M – n(107) salt 108 n This is … CHAPTER 5: MOLECULAR ORBITALS NO+. Bond order = 3 shortest bond (106 pm). NO Bond order = 2.5 intermediate (115 pm) ... molecular orbitals in the diagram suggest a double bond.29 pages
lavelle.chem.ucla.edu › forum › viewtopicMolecular Orbital Diagram of NO+ - CHEMISTRY COMMUNITY Molecular Orbital Diagram of NO+ Postby Josh Ku 3H » Tue Nov 15, 2016 7:23 pm viewtopic.php?f=43&t=16787 In the link above chem_mod said it was best to account for the negative charge of CN- by placing an extra electron on the nitrogen since it is more electronegative. I was just wondering if the same applied for molecules with a positive charge.
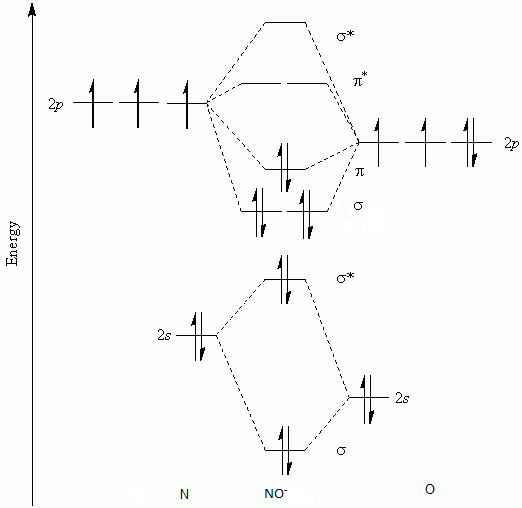
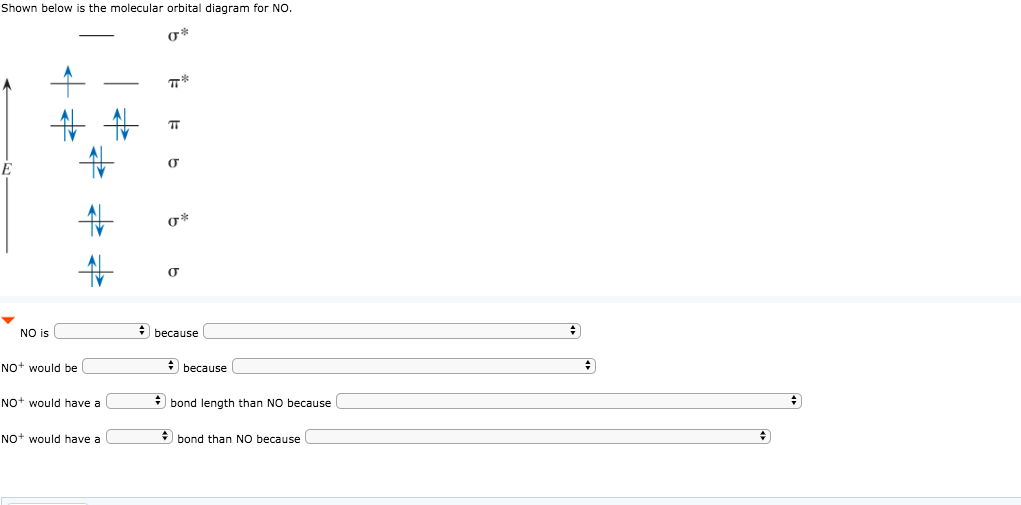
Answered: Draw the molecular orbital diagrams for… | bartleby Draw the molecular orbital diagrams for NO, NO+, and NO-. For each molecule, determine the bond order, if the molecule is stable, and if the molecule is stable if it is paramagnetic or diamagnetic. Rank the molecules in increasing order of bond strength.
› homework-help › draw-molecularSolved: Draw the molecular orbital diagrams for NO- and NO+ ... 115E Draw the molecular orbital diagrams for NO - and NO +. Compare the bond orders in these two ions. Step-by-step solution Step 1 of 4 Molecular orbitals are formed by linear combination of atomic orbitals. Atomic orbitals and molecular orbitals of a molecule can be shown in a molecular orbital diagram. Chapter 10, Problem 115E is solved.
Question: How To Draw Molecular Orbital Picture ... Does SP mixing occur in no+? The phenomenon of s-p mixing occurs when molecular orbitals of the same symmetry formed from the combination of 2s and 2p atomic orbitals are close enough in energy to further interact, which can lead to a change in the expected order of orbital energies.
PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
computational chemistry - What is the dipole moment ... While we see the same polarisation of the molecular orbitals as in carbon monoxide, the magnitude differs and the HOMO of $\ce{NO+}$ cannot compensate for the polarisation of the electron density towards the oxygen.
energy - Molecular orbital diagram for nitrogen monoxide ... It is probably safe to assume that the orbitals of π symmetry don't split that much and are higher in energy than the σ lone pair. Below you can find the calculated MO. The level of theory used for N O X + was RMP2/def2-QZVPP, because this is equivalent to the level the other molecules were treated, which is ROMP2//UMP2/def2-QZVPP.
NO, NO+ and NO-. Using the molecular orbital theory, how ... Jun 9, 2018 — Draw three MO diagrams for NO. Figure out how many electrons are in each compound and fill them in. By comparing the number of electrons in bonding and anti ...5 answers · Top answer: NO+ has 10 valence electrons: (Sigma2s)^2(Sigma*2s)^2(Pi2px,Pi2py)^4(Sigma2pz)^2 NO has 11 ...What is the molecular orbital diagram for NO? - Quora2 answersAug 4, 2018Why is NO+ more stable than NO with a help of the ...3 answersJul 7, 2018More results from
Bond Order Formula: Definition, Calculation, Problems - Embibe Jan 28, 2022 · Molecular Orbital Theory. Molecular orbitals are formed by the combination of atomic orbitals of the bonded atoms. For example, the formation of the hydrogen molecule. A hydrogen molecule is formed by the combination of atomic orbitals of hydrogen \({\rm{A}}\) and \({\rm{B}}\) Each hydrogen atom has one electron in \({\rm{ 1s }}\) orbital.
Solved 1a) Draw the molecular orbital diagram of NO, place ... 1a) Draw the molecular orbital diagram of NO, place the electrons in the atomic and molecular orbitals, label the atomic and molecular orbitals 1b) How many electrons are placed in the π* orbitals of NO, NO+ and NO- 1c) Determine the spin states S for NO, NO+ and NO- . two spin states may exist for NO-, what are they? Describe spin state in terms
socratic.org › questions › 59a5709a11ef6b7ac40bc96dUsing the MO diagram of #"NO"#, calculate the ... - Socratic.org Chemistry Molecular Orbital Theory Molecular Orbital Theory 1 Answer Truong-Son N. Aug 29, 2017 The MO diagram for NO is as follows (Miessler et al., Answer Key): (The original was this; I added the orbital depictions and symmetry labels. For further discussion on the orbital energy ordering being N2 -like, see here and comments.)
Cyanide Molecular Orbital Diagram - schematron.org A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. In this answer of Martin's, you can find a molecular orbital diagram of $\ce {CO}$.
Essentials of Physical Chemistry by B.S ... - Academia.edu Preface The Essentials of Physical Chemistry has been written for BSc students. It has been national bestseller for more than 65 years. It has been used by more than 2 million students. It is 26 editions old. It really has been that long. A lot of
Consider the following molecules: NO, NO+ and NO-. Using ... Answer (1 of 5): NO+ has 10 valence electrons: (Sigma2s)^2(Sigma*2s)^2(Pi2px,Pi2py)^4(Sigma2pz)^2 NO has 11 valence electrons: Same as NO+ but add (Pi*2px,Pi*2py)^1 NO- has 12 valence electrons: Same as NO but change ^1 at the end to ^2. Your MO diagram for NO should look like this: O is m...
modernizemodest1712.blogspot.com › 2022/02/37-no37 no+ molecular orbital diagram - Diagram For You Feb 01, 2022 · No+ molecular orbital diagram Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals.
a = Number of electrons in bonding molecular orbitals. b = Number of electrons in antibonding molecular orbitals. (1) NO+ Total number of electrons in NO + = 14 Molecular configuration can be written as (σ1s) 2 (σ*1s) 2 (σ2s) 2 (σ*2s) 2 (π) 4 (2p z) 2 a = 10 b= 4 Bond order = 1/2 (10 - 4) Bond order = 1/2 (6) Bond order = 3 Bond order of NO+ is 3.
According to the molecular orbital energy-level diagram ... The untrue statement among the options is that only one of the species is paramagnetic.. In the molecular orbital model, electrons could be filled into molecular orbitals according to the Aufbau principle in the same way electrons can be filled into atomic orbitals.. The molecular orbital configuration of the species listed are as follows;. NO = (σ2s)2 (σ⋆2s)2 (π2px)2 (π2pz)2 (σ2pz)2 ...
MOT(VI)NO, NO+&NO- - YouTube ¤ Molecular Orbital diagram of BN, CN, CN- ¤Molecular Orbital diagram of COhttps://youtu.be/8mufOTgvagU¤ S-P mixing OF ORBITALS ...





















0 Response to "45 no+ molecular orbital diagram"
Post a Comment