44 use the reaction energy diagram above to answer the following questions.
Using the diagram above, answer the following questions: 6 ... Using the diagram above, answer the following questions: 6. True or False. The arrow labeled C represents a transfer of chemical energy to mechanical energy. Explain why this is true or false. 7. True or False. The arrow labeled A represents a transfer of solar energy to chemical energy. Explain why this is true or false. 8. PDF CHAPTER 7 QUESTIONS Multiple-Choice Questions Gaseous hydrogen and fluorine combine in the reaction above to form hydrogen fluoride with an enthalpy change of -540 kJ. What is the value of the heat of formation of HF(g)? (A) -1,080 kJ/mol (B) -270 kJ/mol (C) 270 kJ/mol (D) 540 kJ/mol Use the following information to answer questions 20-23. When calcium chloride (CaCl 2
PDF CHAPTER 6 QUESTIONS Multiple-Choice Questions 6. Use your knowledge of kinetics to answer the following questions. Justify your answers. (a) Potential Energy 1 2 Reaction Coordinate The two lines in the diagram above show different reaction pathways for the same reaction. Which of the two lines shows the reaction when a catalyst has been added? (b) Fractions of Molecules 1 2 Energy Which ...
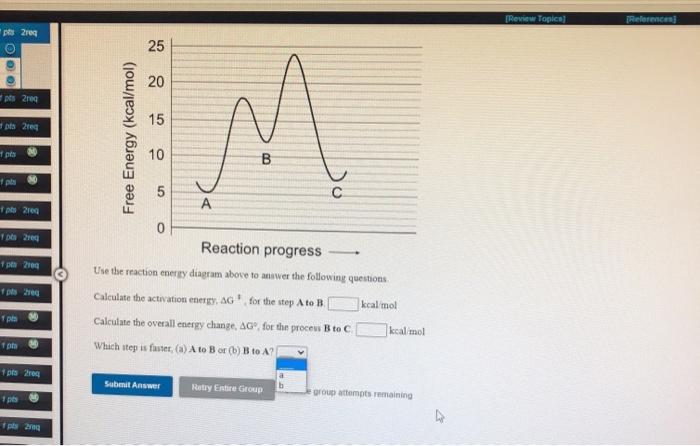
Use the reaction energy diagram above to answer the following questions.
⚗️Using the diagram above, answer the following questions ... Jan 20, 2021 · Using the diagram above, answer the following questions: 6. True or False. The arrow labeled C represents a transfer of chemical energy to mechanical energy. Explain why this is true or false. – 7. True or False. The arrow labeled A represents a transfer of solar energy to chemical energy. Explain why this is true or false. – 8. Free Energy Practice Exam Questions Flashcards | Quizlet Match. Gravity. Refer to the free energy diagrams below to answer the following questions. You may assume that the y-axis is the same and directly comparable for all four reactions. Click card to see definition 👆. Tap card to see definition 👆. Potential Energy Diagrams | Chemistry for Non-Majors A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction. This ...
Use the reaction energy diagram above to answer the following questions.. PDF Kinetics Free Response Sample Questions Kinetics - Free Response Sample Questions 2005 B Answer the following questions related to the kinetics of chemical reactions. I- (aq) + ClO- (aq) O H- o IO (aq) + Cl- (aq) Iodide ion, I-, is oxidized to hypoiodite ion, IO-, by hypochlorite, ClO-, in basic solution according to the equation above. Use the reaction energy diagram above to answer the followin Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ?G, for the step D to C. _____ kcal/mol. Calculate the overall energy change, ?G°, for the process B to A. _____ kcal/mol PDF AP CHEMISTRY 2009 SCORING GUIDELINES (Form B) Answer the following questions about nitrogen, hydrogen, and ammonia. (a) In the boxes below, draw the complete Lewis electron-dot diagrams for N 2 and NH 3. The correct structures are shown in the boxes above. Two points are earned for the correct Lewis electron-dot diagrams (1 point each). Answered: 25 20 15 10 Reaction progress Use the… | bartleby 25 20 15 10 Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, AG, for the step B to C. kcal/mol Calculate the overall energy change, AG°, for the process A to B. kcal/mol Which step is faster, (a) C to B or (b) B to A? Free Energy (kcal/mol) B.
DOC Review Answers Answer the following questions regarding the kinetics of chemical reactions. a. The diagram to the right shows the energy pathway for the reaction O3 + NO → NO2 + O2. Clearly label the following directly on the diagram. i. The activation energy (Ea) for the forward reaction. ii. The enthalpy change (ΔH) for the reaction. b. Free Energy (kcal/mol) Reaction progress- Use the reaction ... 25 20 15 Free Energy (kcal/mol) M B 10 r 5 0 Reaction progress Use the reaction energy diagram above to answer the following questions, Calculate the activation energy. AG #, for the step C to B. Include algebraic sign. Calculate the overall energy change, AG®, for the process to D. Include algebraic sign. How to draw the potential energy diagram for this reaction ... 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process. Using the diagram above, the following questions: True or ... Using the diagram above, answer the following questions: True or False. The arrow labeled C represents a transfer of chemical energy to mechanical energy. Explain why this is true or false. True or False. The arrow labeled A represents a transfer of solar energy to chemical energy. Explain why this is true or false.
PDF (b) rate = k [X]2 - WOU Use the following information to answer the next two questions 3. For the chemical reaction system described by the diagram above, which statement is true? (a) The forward reaction is endothermic. (b) The activation energy for the forward reaction is greater than the activation energy for the reverse reaction. (c) At equilibrium, the activation ... [Solved] 25 Free Energy (kcal/mol) 20 15 10 B A C D O ... 25 Free Energy (kcal/mol) 20 15 10 B A C 0 Reaction progress - Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, AG * , for the step B to A. kcal/mol Calculate the overall energy change, AG, for the process C to B. kcal/mol Which step is faster, (a) A to B or (b) C to B? Instructions: Use the reaction energy diagram below to ... Instructions: Use the reaction energy diagram below to answer the following question(s).-The following group is a substituent on a molecule. What is an accepted IUPAC name for this group? A) propenyl B) allyl C) vinyl D) propylene E) either a or b PDF KM 554e-20161110155017 - Weebly Reaction Il is endothermic, and the activation energy of reaction I is greater than that of reaction O Reaction Pathway (a) Complete the potential-energy diagram for reaction Il on the graph above.. (b) For reaction I, predict how each of the following is affected as the temperature is increased by 200C. Explain the basis for each prediction.
OneClass: #1 #2#3 25 E 20 é 15 C D 0 Reaction progress Use ... 25 E 20 é 15 C D 0 Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, Î G , for the step A to B. Calculate the overall energy change, AGo, for the process B to C.
Chemistry Unit 6 Flashcards - Quizlet Use the potential energy diagram below to answer the following question The potential energy of the reactants is ____kJ, while the potential energy of the products is _____. ( straight, wayyyyyyyyyyy uphill, then tiny downhill, then straight )
Answered: 25 20 15 B 10 A C Reaction progress Use… | bartleby Free Energy (kcal/mol) 25 20 15 B 10 A C Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, AG *, for the step C to B. kcal/mol Calculate the overall energy change, AG°, for the process B to D. kcal/mol Which step is faster, (a) B to A or (b) A to B? Free Energy (kcal/mol)
Use the potential energy diagram shown (se... | Clutch Prep Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
Topic 5.6 WRSHT.pdf - Topic 5.6 Reaction Energy Profile ... Topic 5.6 Reaction Energy Profile Worksheet Use the following potential energy diagram to answer questions 1-7 below: 1. Is the overall reaction, as shown, exothermic or endothermic? 2. Which letter represents the activation energy for the forward reaction? 3. Which letter represents the activation energy for the reverse reaction? 4.
Solved Use the reaction energy diagram above to answer the ... Chemistry questions and answers. Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ?G, for the step C to D. kcal/mol Calculate the overall energy change, ?G°, for the process A to B. Question: Use the reaction energy diagram above to answer the following questions.
1- Use the reaction to complete the sentence. 2CO + O2 ... a is one of the reactants of the reaction. b provides the kinetic energy for the reaction c is one of the products of the reaction. d provides the activation energy for the reaction. 8- Use the diagram to answer the question. A diagram shows the energy levels of hydrogen chloride and molecules of hydrogen and chlorine.
PDF Potential Energy Diagram Worksheet ANSWERS Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what determines the rate at which the reaction occurs?
PDF Chemical kinetics Name: Date - The Leon M. Goldstein High ... On the diagram below, draw a potential energy diagram for this reaction. 18. Base your answer(s) to the following question(s) on the reaction represented by the balanced equation below. 2H2(g)+O2(g) !2H2O(')+571:6kJ On the axes below, draw a potential energy diagram for the reaction represented by this equation. 19. Given the reaction at ...
Use the reaction energy diagram above to a... | Clutch Prep Problem: Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ΔG for the step C to B _____ kcal/mol Calculate the overall energy change, ΔG°, for the process B to A. _____kcal/mol.
Application and Practice Questions - Physics Classroom Friction would do negative work and thus remove mechanical energy from the falling ball. Use the following diagram to answer questions #3 - #5. Neglect the effect of resistance forces. 3. As the object moves from point A to point D across the surface, the sum of its gravitational potential and kinetic energies ____.
PDF CEM 143 1) In the space provided draw the structure of ... 2) Name the following compound (3 pts.) OH Part 2. Use the reaction energy diagram to answer the following questions OH Products 3) What type of substitution reaction could be represented by the above reaction energy diagram? (3pts.)
Potential Energy Diagrams | Chemistry for Non-Majors A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction. This ...
Free Energy Practice Exam Questions Flashcards | Quizlet Match. Gravity. Refer to the free energy diagrams below to answer the following questions. You may assume that the y-axis is the same and directly comparable for all four reactions. Click card to see definition 👆. Tap card to see definition 👆.
⚗️Using the diagram above, answer the following questions ... Jan 20, 2021 · Using the diagram above, answer the following questions: 6. True or False. The arrow labeled C represents a transfer of chemical energy to mechanical energy. Explain why this is true or false. – 7. True or False. The arrow labeled A represents a transfer of solar energy to chemical energy. Explain why this is true or false. – 8.

0 Response to "44 use the reaction energy diagram above to answer the following questions."
Post a Comment