44 lewis dot diagram for methane
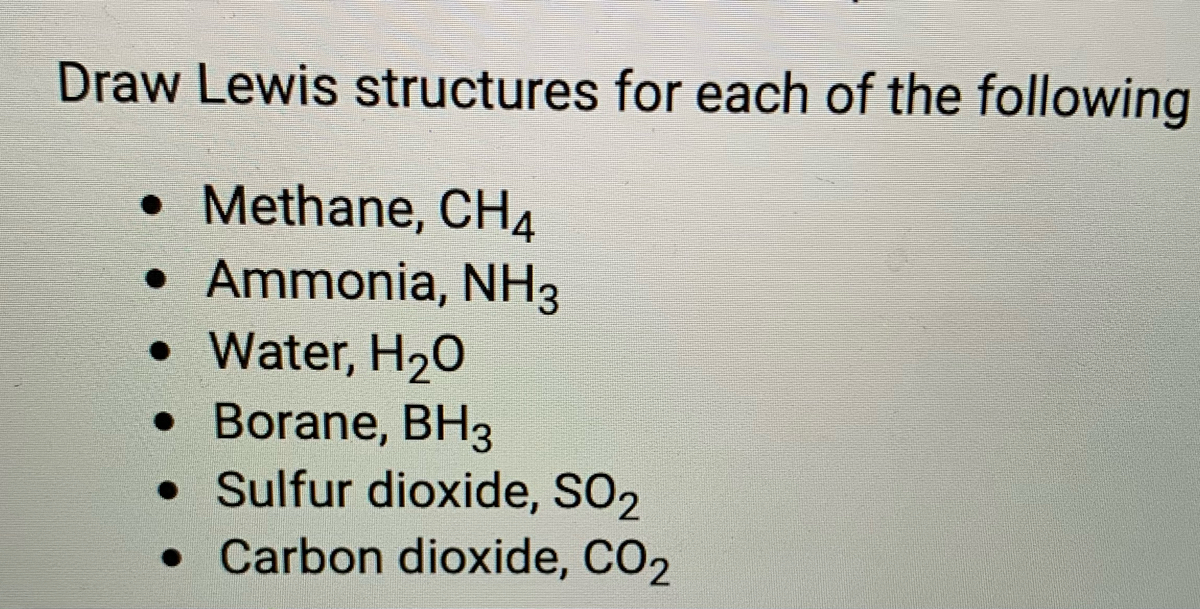
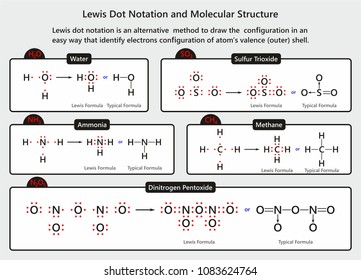
Ch4 Electron Dot Diagram - schematron.org Lewis Dot Structure for CH4 #2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 . We show two ways to draw the CH4 Lewis structure, methane. We also have This info can then be used to determine the Lewis Dot Structure. Methane Cross And Dot Diagram - schematicstar.com Now, let's know its structure: Lewis dot structure is a representation of. (c) The dot-and-cross diagram of BF3 is *7 The compounds hydrogen fluoride, water and methane, all have simple molecular.Answer: Well Carbon only has 4 valence electron, so it can bond at all four point.
How to Draw the Lewis Structure of CH4 (methane) - YouTube Check me out:

Lewis dot diagram for methane
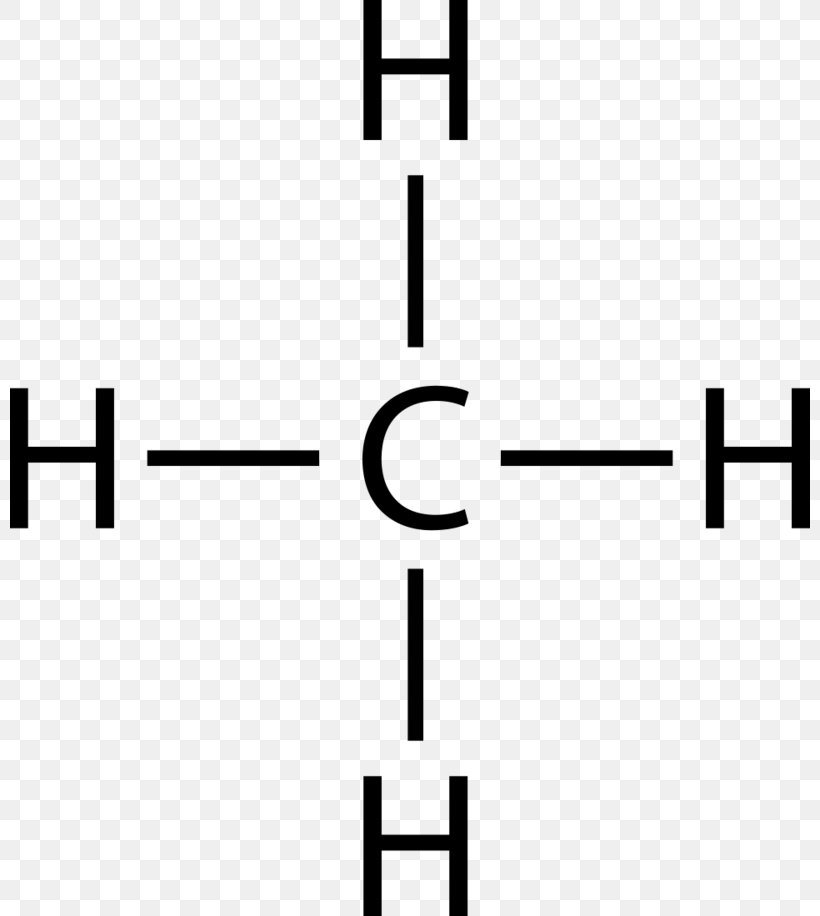
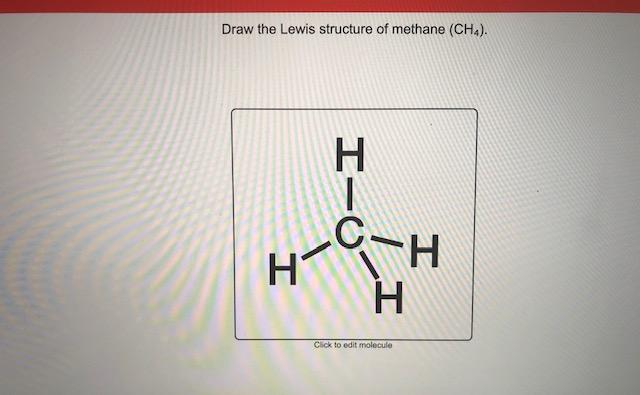
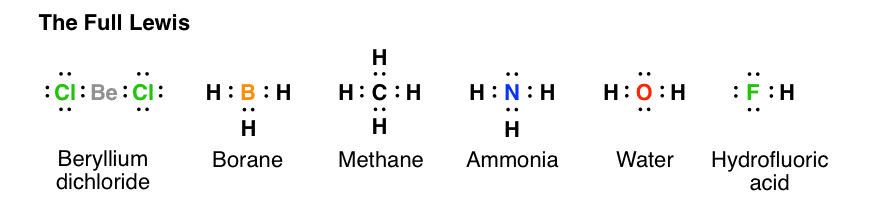
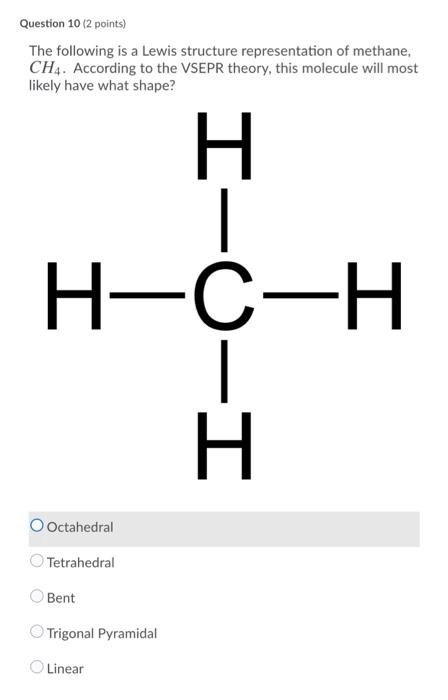
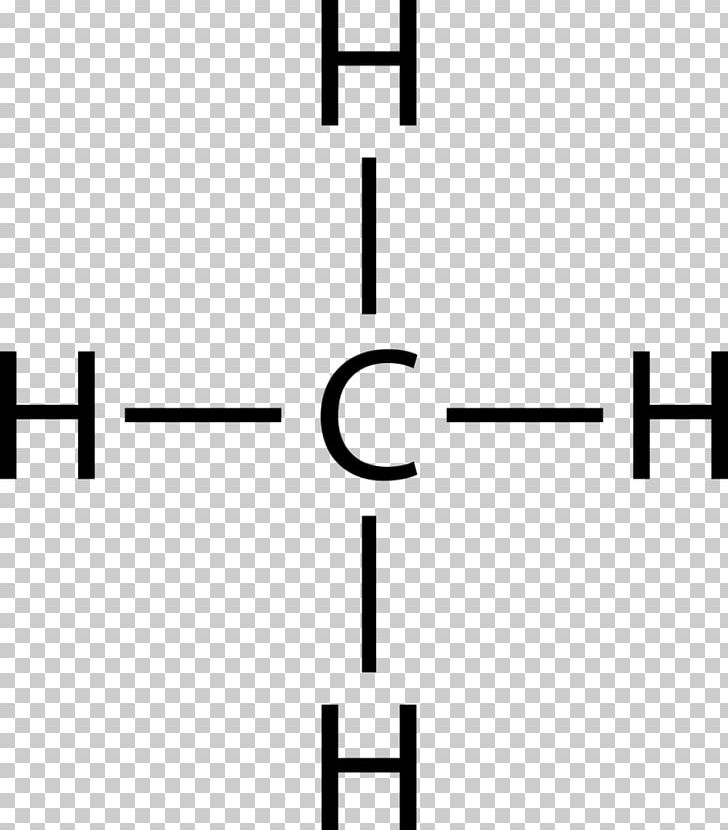
Lewis Dot Structure for CH4- Methane - Bob Cut Magazine This is the Lewis Dot representation of methane. By representing each of the bonds with one line, these types of structures can also be illustrated. Each atom in the bond has a complete valence, with eight electrons being accessed by carbon and two accessed by each hydrogen. CH4 Lewis Structure - How to Draw the Dot Structure for ... How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use... Lewis Structure of CH4 (Methane), Shape & Hybridization Lewis structure of methane shows that the central atom C has four bonding electron pairs. These electron pairs repel each other and are thus directed to the four corners of a regular tetrahedron. A regular tetrahedron is a solid figure with four faces which are equilateral triangles. All bond angles are 109.5° .
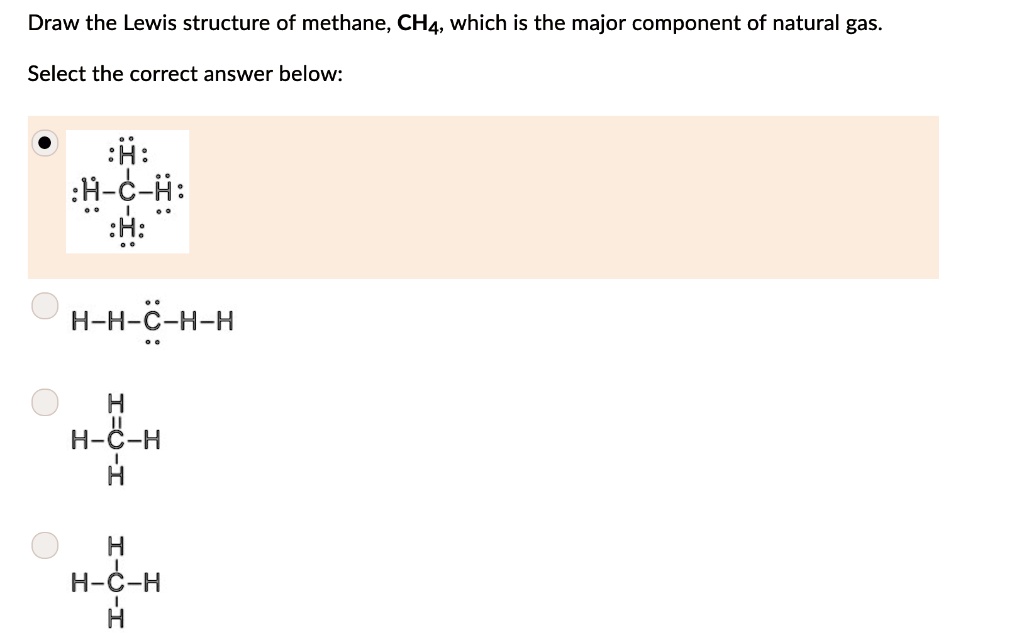
Lewis dot diagram for methane. Electron Dot Diagram For Methane - schematron.org Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) . Lewis dot dragram for methane: Methane, with molecular formula CH4, is shown. It is important to remember that Lewis valence dot diagrams are models that Methane is the main component of natural gas, and its chemical formula is CH4. Lewis Dot Diagram Ch4 Drawing the Lewis structure for CH 4 (named methane) requires only single diagramweb.net's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. Lewis dot structure of CH 4. How to draw the lewis dot structure of Methane (CH4) - YouTube Step by step explanation showing how to draw the Lewis structure of Methane (CH4). Lewis Dot Structure for CH4 | Chemical Bonding | Electron ... Chemical Bonding: Lewis Dot Structure for CH4(2 of 6) Dr. B. explains how to draw the Lewis dot structure for CH4(methane). The CH4Lewis Structure is one of the most frequently tested Lewis Structures. Note that hydrogen atoms always go on the outside of a Lewis dot structure. This is because they can share a maximum of two electrons.
Lewis Dot Structures 1. Methane 4 - HCC Learning Web Methane 4 Lewis Dot Structures 1. Methane - CH 4 Number of Valence Electrons: 4 from C and 1 each from 4 H = 8 Carbon is more electronegative than hydrogen, but hydrogen can never be the "central" atom, as it can only form 1 bond. Carbon always forms 4 bonds (2 electrons each). 2. Ammonia - NH 3 Lewis Structure for CH4 (Methane) - UMD Drawing the Lewis structure for CH 4 (named methane) requires only single bonds. It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. Video: Drawing the Lewis Structure for CH4 Dot Diagram For Ch4 - Wiring Diagram Pictures But seriously, you have an electron pair between the C and each of the H's in the Lewis. Dr. B. explains how to draw the Lewis dot structure for CH 4 (methane). The CH 4 Lewis Structure is one of the most frequently tested Lewis Structures.. Note that hydrogen atoms always go on the outside of a Lewis dot structure. How to Draw the Lewis Dot Structure for CH4: Methane - YouTube A step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use the periodic table to find the total number of vale...
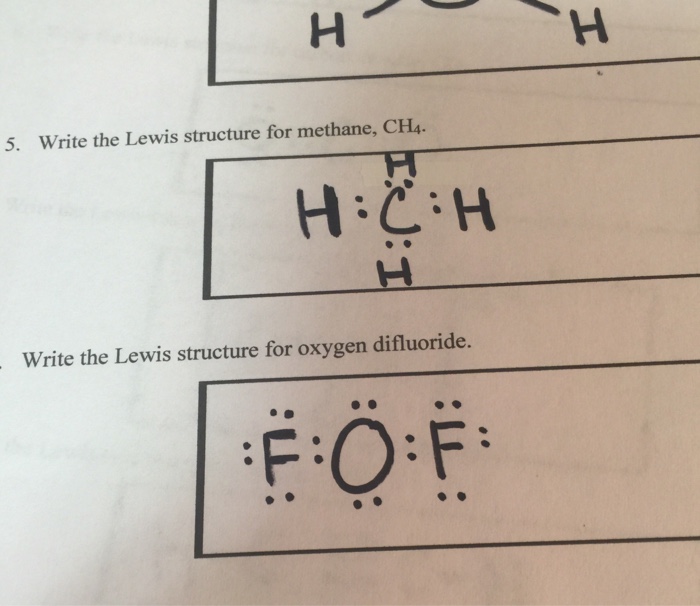
Lewis dot structure of methane - fornoob.com Lewis dot structure of methane. December 27, 2021 thanh. below is the Lewis structure of chlorine. Below is the Lewis structure of the methane (CH_4) molecule Count the number of bonding pairs and the number of lone pairs around the carbon atom. CH4 Lewis Structure, Molecular Geometry, and Hybridization The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3. Methane (CH4) lewis dot structure, molecular geometry ... According to the lewis dot structure of CH4, the Carbon central atom doesn't contain any lone pair but is attached to the four hydrogen atoms with the help of four bonded pairs. Summary The total valence electron available for drawing the Methane (CH4) lewis structure is 8. Cargill Rail Yard. In Chapter 14 we continue our study of ... While previously we drew a Lewis structure of methane Transcript. CH3OCH2CH3 is methoxyethane. The initial. Even if the Hydrogens and Chlorines switch places, the molecule will retain its structure. Which of the following statements about Lewis structures is false? When you draw a Lewis structure for CH3OCH2CH3, what is the total number of lone ...
Methane CH4 Lewis Dot Structure - YouTube A video explanation of how to draw the Lewis Dot Structure for Methane, along with information about the compound including Formal Charges, Polarity, Hybrid ...
Lewis Dot Structure of CH4 (methane) - YouTube I quickly take you through how to draw the Lewis Structure of methane, CH4. I also go over hybridization, shape and bond angle.
Draw Lewis dot diagram for the following. Methane (CH4 ... Diagram. Draw Lewis dot diagram for the following. Methane (CH 4) Advertisement Remove all ads.
What is the Lewis dot structure of methane? - Answers No, not exactly. It is an ionic compound so it would not have a Lewis dot structure. However, the carbonate anion, CO3^2- does have a Lewis dot structure.
Lewis Structure of CH4 (Methane), Shape & Hybridization Lewis structure of methane shows that the central atom C has four bonding electron pairs. These electron pairs repel each other and are thus directed to the four corners of a regular tetrahedron. A regular tetrahedron is a solid figure with four faces which are equilateral triangles. All bond angles are 109.5° .
CH4 Lewis Structure - How to Draw the Dot Structure for ... How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use...
Lewis Dot Structure for CH4- Methane - Bob Cut Magazine This is the Lewis Dot representation of methane. By representing each of the bonds with one line, these types of structures can also be illustrated. Each atom in the bond has a complete valence, with eight electrons being accessed by carbon and two accessed by each hydrogen.


![Expert Verified] Draw electron dot structure of methane and ...](https://hi-static.z-dn.net/files/d9a/d2894676bfa85a26ef3221bdb07b9a0d.jpg)




















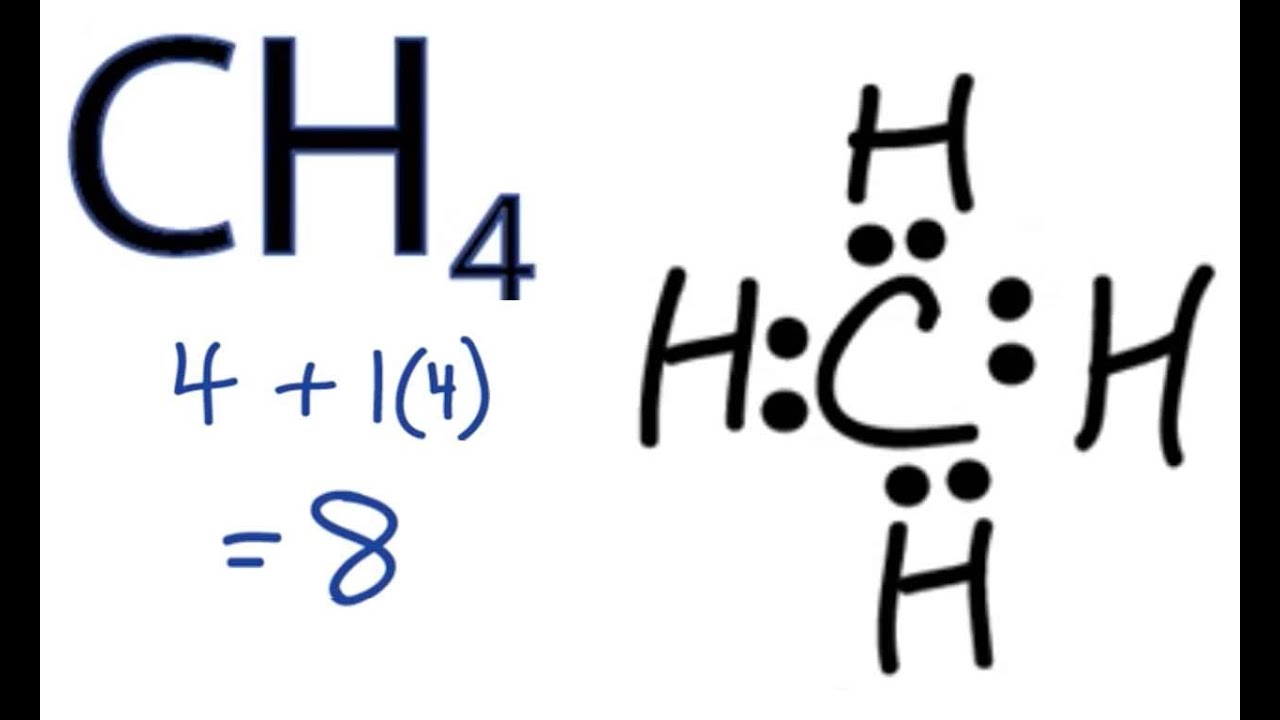






0 Response to "44 lewis dot diagram for methane"
Post a Comment