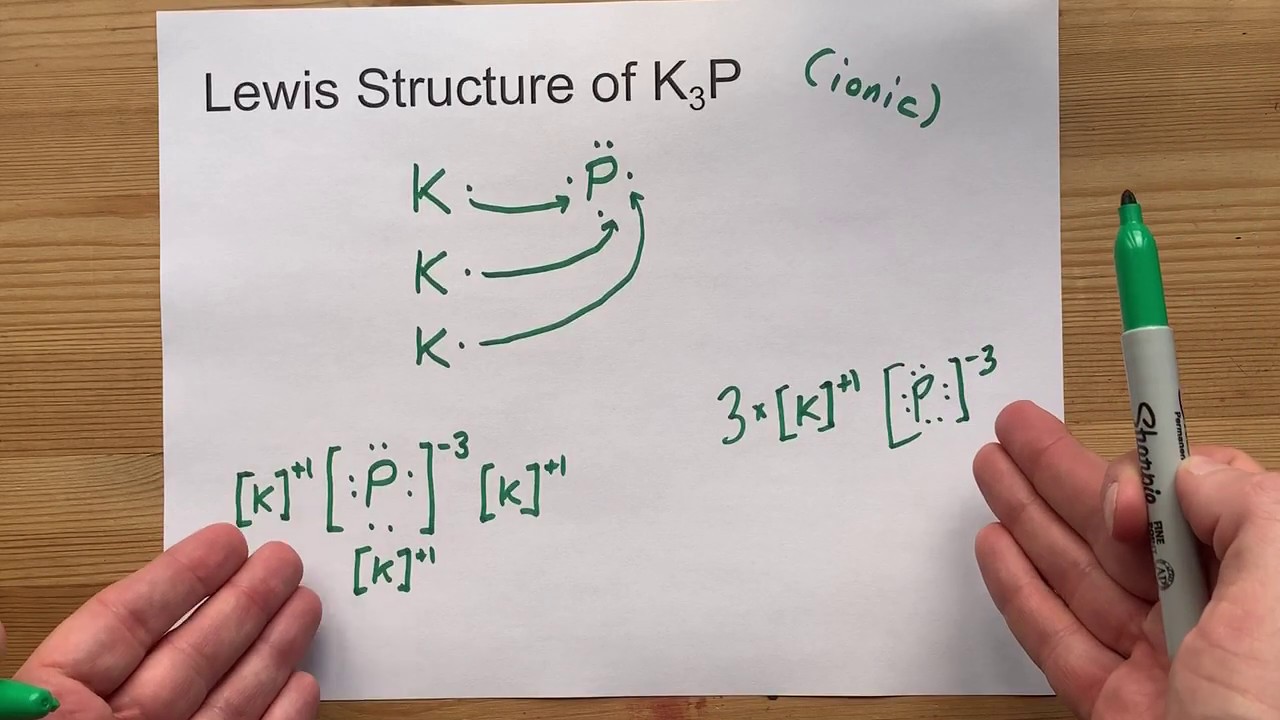
44 electron dot diagram for phosphorus
Lewis dot diagram for phosphorus? - Answers It is Triangular Bipyrcamidal with a SP3d hybridization. A Lewis dot diagram would be drawn as phosphorus as the central atom, then the 5 fluorines surrounding the central atom. What is the electron dot notation for phosphorus? - Answers The dots represent the electrons in valence shell. Phosphorus= P it has five electrons on the outer shell. There are three dots around unpaired while the dots on the right of the P are paired.
Lewis Electron Dot Diagrams - lardbucket Electron dot diagrams for ions are the same as for atoms, except that some electrons have been removed for cations, while some electrons have been added for anions. Thus in comparing the electron configurations and electron dot diagrams for the Na atom and the Na + ion, we note that the Na atom has a single valence electron in its Lewis diagram ...
Electron dot diagram for phosphorus
Solved a) Draw a valid lewis electron dot Structure for ... (A) There are 26 valence electrons to be used when drawing the Lewis dot structure for Phosphorous trichloride; 5 from phosphorus as it occurs in the 15th column of the Periodic Table and 7 from each of the chlorine atoms as chlorine occurs in the 17… View the full answer How many unpaired electrons are in a phosphorus atom ... There are three unpaired electrons. As you can see in the electron configuration, the 3p sublevel has room for three more electrons. The orbit filling diagram and the electron dot diagram show the empty spaces for three more electrons and how there are three electrons that aren't paired. The electron dot diagram for an unknown element X is shown ... D - Silicon. This is because Silicon has four electrons as indicated by the group it's in. Chlorine would have 7, Magnesium would have 2, Phosphorous would have 5, and Sulfur would have 6. Hope this helps!
Electron dot diagram for phosphorus. Lewis Dot Diagram For Phosphorus - schematron.org More information about the DOT ID/UN number and the guide number can be found at the Emergency Response Guidebook. Nov 22, · Best Answer: The Lewis Electron Dot Diagram for Phosphorous is P with 5 dots around it. There are 5 valence electrons, so this is how many dots are around. Each dot represents one electron. Electron Configuration for Phosphorus (P) - UMD The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 3. Lewis Dot Diagram Phosphorus - wiringall.com Nov 22, · Best Answer: The Lewis Electron Dot Diagram for Phosphorous is P with 5 dots around it. There are 5 valence electrons, so this is how many dots are around. Each dot represents one electron. When it bonds with another atom, it needs to have 8 total electrons to complete the valence wiringall.com: Resolved. The Lewis dot diagram for Platinum is a diagram showing bonds & electrons of the Platinum atom within a molecule. opentextbc.ca › lewis-electron-dot-diagramsLewis Electron Dot Diagrams – Introductory Chemistry – 1st ... The electron dot diagram for helium, with two valence electrons, is as follows: By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1 s subshell; this is the common convention we will adopt, although there will be exceptions later.
Electron Dot Diagrams | Chemistry for Non-Majors In the p block, the number of valence electrons is equal to the group number minus ten. Group 13 has three valence electrons, Group 14 has four, up through Group 18 with eight. The eight valence electrons, a full outer s and p sublevel, give the noble gases their special stability. Phosphorus Dot diagram - Summarized by Plex.page | Content ... Chemical bonds are created in the majority of cases by chemical reactions of valence electrons in atoms. Lewis diagrams, Lewis dot formulas, Lewis dot formulas, Lewis dot designs, electron dot structures, Lewis electron dot structures, or Lewis electron dot structures are diagrams that illustrate the bonding between atoms of a molecule, as well as the molecule's lone pairs of electrons. How to Draw the Lewis Dot Diagram of P (Phosphorus ... How to Draw the Lewis Dot Diagram of P (Phosphorus) - YouTube. Lewis Dot Diagram For Lithium And Phosphorus - Novocom.top lewis dot phosphorus diagram electron structure pcl3 pairs electrons lone chlorine cl valence bond distribute each
Lewis Dot Diagram Magnesium And Phosphorus - Novocom.top dot lewis electron diagram diagrams krypton boron phosphorus structure sodium titanium draw mg element br fe atom courses chemintro peoi bromide phosphorus fluorescence sirna ethidium lewis structure phosphorus pi3 triiodide draw sciencing.com › calculate-electron-configurationHow to Calculate Electron Configuration | Sciencing Apr 24, 2017 · To calculate the electron configuration for phosphorus (P), which is in the third row, p-block, third element in that block, write: 1s2 2s2 2p6 3s2 3p3. Check your work by adding the electron numbers to see if they equal the atomic number of the element; for this example, you would write: 2+2+6+2+3=15, which is the atomic number of phosphorus. What is the Lewis dot structure for P? - FindAnyAnswer.com The electron dot or Lewis dot structure of P4,which is the constituent molecule of white phosphorus,can be easily drawn keeping in mind the facts that: 1)It has tetrahedral geometry. 2)Each P has 5 valence e-s and thus in P4 there are 5×4=20 valence e-s. What is the Lewis dot structure for PCl3? - handlebar ... We can clearly see from the lewis diagram that in PCl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. With that 2 lone pairs are present on the phosphorus atom. This concept very well explains the hybridization of PCl3 which is sp3.
PDF Name: Bonding Review - Welcome to Dr. Mintz's Chemistry ... 9.Given the electron dot diagram: 1)hydrogen, which has the higher electronegativity 2)fluorine, which has the higher electronegativity 3)hydrogen, which has the lower electronegativity 4)fluorine, which has the lower electronegativity The electrons in the bond between hydrogen and fluorine are more strongly attracted to the atom of 1)strontium ...
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, ...
How would you draw a Lewis structure for an ... - Socratic.org Phosphorus has 5 valence electrons. To draw Lewis Structures for elements, the symbol for the element is drawn with the number of valence electrons it has surrounding it. So, to draw the Lewis Structure, begin by drawing the symbol for Phosphorus, the letter P. Next, Phosphorus has 5 valence electrons. So start with one dot on top, then one dot to the right, one dot on the bottom, one dot to the left, and another dot on top, next to the first one.
Phosphorus Bohr Model - How to draw Bohr diagram for ... Electron dot diagram of a Phosphorus atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Phosphorus, we got to know, it has 5 valence electrons. So, just represent the 5 valence electrons around the Phosphorus atom as a dot.
study.com › academy › answerDraw the Lewis structure for CH3CH2Cl and provide the ... b. number of shared electron pairs around the central atom ... Review what a Lewis dot diagram is and discover how to draw a Lewis dot structural formula for compounds. ... Consider the phosphorus ...
Electron Dot Structure For Phosphorus - Summarized by Plex ... The number of dots equals the number of valence electrons in an atom. These dots are arranged to right and left and above and below the symbol, with no more than two dots on side. For example, Lewis electron dot diagram for calcium is simply figure 1 shows Lewis symbols for elements of the third period of the periodic table.
topblogtenz.com › phosphorus-trifluoride-pf3-lewisPhosphorus trifluoride (PF3) lewis dot structure, molecular ... In this article, we will discuss Phosphorous trifluoride (PF3) lewis dot structure, molecular geometry, electron geometry, hybridization, polar or nonpolar, its bond angle, etc. “Phosphorus trifluoride is similar to carbon monoxide in that it is a gas which strongly binds to iron in hemoglobin, preventing the blood from absorbing oxygen.”
What is the Lewis electron dot diagram for phosphorus ... A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
PDF Electron Dot (Lewis) Diagrams - Mr. Sault's Classroom Electron Dot Diagrams There is another model called the electron dot or Lewis diagram. This system represents an atom and its valence electrons. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons. The electrons in the valence shell are shown as dots placed around the symbol.
topblogtenz.com › sodium-bohr-modelSodium Bohr Model - How to draw Bohr diagram for Sodium(Na) atom Electron dot diagram of a Sodium atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Sodium, we got to know, it has only 1 valence electron. So, just represent the 1 valence electrons around the Sodium atom as a dot.
What is the electron dot diagram for calcium? - AskingLot.com A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. The total number of electrons does not change.
PO43- Lewis Structure (Phosphate ion) PO 4 3-Lewis Structure (Phosphate ion). Lewis structure of phosphate ion is drawn clearly in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of PO 4 3-ion. In lewis structure, there should be charges on atoms.
qualifications.pearson.com › content › damMark Scheme (Results - Edexcel Aug 22, 2018 · electron(s) and S gains electron(s) No M1 or M2 if mention of electron sharing or covalent bonding ALLOW Mg (ion) has a charge of 2+/+2 and S (ion) has a charge of 2-/-2 Two correct ionic half equations scores all 3 marks 3 Diagrams showing electron transfer and charges on the ions scores all 3 marks
en.wikipedia.org › wiki › Octet_ruleOctet rule - Wikipedia Other rules exist for other elements, such as the duplet rule for hydrogen and helium, or the 18-electron rule for transition metals. The valence electrons can be counted using a Lewis electron dot diagram as shown at the right for carbon dioxide.
The electron configuration for phosphorous is ... Answer: See the picture attached for the Lewis electron dot diagram for phosphorus.; Explanation: The Lewis electron diagram is a representation of the atoms that shows the the valence electrons present in the atom.. The Lewis electron diagram uses the chemical symbol of the element and adds one dot for every valence electron.
Lewis Dot Diagram Phosphorus - schematron.org Example: Draw the Lewis structure for phosphorus pentafluoride, PF 5. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
The electron dot diagram for an unknown element X is shown ... D - Silicon. This is because Silicon has four electrons as indicated by the group it's in. Chlorine would have 7, Magnesium would have 2, Phosphorous would have 5, and Sulfur would have 6. Hope this helps!
How many unpaired electrons are in a phosphorus atom ... There are three unpaired electrons. As you can see in the electron configuration, the 3p sublevel has room for three more electrons. The orbit filling diagram and the electron dot diagram show the empty spaces for three more electrons and how there are three electrons that aren't paired.
Solved a) Draw a valid lewis electron dot Structure for ... (A) There are 26 valence electrons to be used when drawing the Lewis dot structure for Phosphorous trichloride; 5 from phosphorus as it occurs in the 15th column of the Periodic Table and 7 from each of the chlorine atoms as chlorine occurs in the 17… View the full answer

0 Response to "44 electron dot diagram for phosphorus"
Post a Comment