43 complete this valence molecular-orbital diagram for oxygen o2
Solved Complete this valence molecular-orbital diagram for ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as needed. 8.4: Molecular Orbital Theory - Chemistry LibreTexts Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
Complete this valence molecular-orbital diagram for oxygen ... Complete the valence molecular-orbital diagram for oxygen, O, Answer Bank. Complete the valence molecular-orbital diagram for oxygen, O, Answer Bank Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click... Construct the molecular orbital diagram for He2 and then identify the bond order.
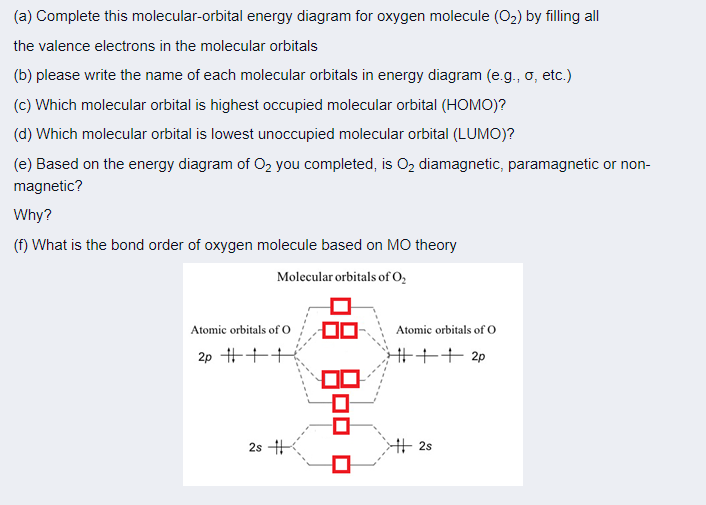
Complete this valence molecular-orbital diagram for oxygen o2
3.3.4: Assembling a complete MO diagram - Chemistry LibreTexts Exercise 3.3.4. 3. Construct a qualitative molecular orbital diagram for chlorine, Cl 2. Compare the bond order to that seen in the Lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). Answer. Construct the molecular orbital diagram fo... | Clutch Prep Construct the molecular orbital diagram for He 2 and then identify the bond order. Click within the blue boxes to add electrons. Bond order: a) 0. b) 0.5. c) 1. d) 1.5. e) 2. Learn this topic by watching MO Theory: Bond Order Concept Videos. molecular orbital diagram for o2 - penguinskiclub.org Penguin Ski Club of New Hampshire. Located in Lincoln NH near Loon Mountain. Menu and widgets
Complete this valence molecular-orbital diagram for oxygen o2. (Get Answer) - Sketch the Molecular Orbital diagram for O2 ... Sketch the Molecular Orbital diagram for O2 being sure to: A. Designate bonding and anti-bonding orbitals, B. Show the location of all electrons in the molecular orbitals, C. Calculate the bond order, and D. Indicate whether this molecule is paramagnetic or diamagnetic. For your diagram, assume that one oxygen atom contributes its six (6 ... Explain the formation of O2 molecule using molecular class ... We know that Oxygen has atomic number = 8. Thus, the electronic configuration for an atom of oxygen in the ground state can be given as - $1{s^2}2{s^2}2{p^4}$ One atom of oxygen has 8 electrons. Thus, two atoms will possess 16 electrons i.e. Oxygen molecules will have 16 electrons. The molecular orbital diagram of an Oxygen molecule is as - PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in Molecular Orbital Theory - Purdue University molecular orbitals are those formed when valence-shell orbitals are combined. The molecular orbital diagram for an O2molecule would therefore ignore the 1selectrons on both oxygen atoms and concentrate on the interactions between the 2sand 2pvalence orbitals. Molecular Orbitals of the Second Energy Level
MO Orbital Lab - Part I: MO diagram of oxygen molecule, O2 ... The valence electrons are in the 2s and 2p orbitals. With 4 electrons placed in the 1s orbital, there are 12 more electrons remaining to be placed in the O2 molecule. The σ and σ* 2s orbital is also completely filled, putting 4 more electrons into the orbital. smartbook 14 Flashcards - Quizlet molecular orbitals can be formed by the combination of multiple atomic orbitals, allowing electrons to be _____ or shared between several atoms. the molecular orbital model therefore allows a better description of the bonding in _____ structure than valence bond theory, which depicts electrons as being _____ between two atoms at a time 42 complete this molecular orbital diagram for cn - Wiring ... Question: Complete the molecular orbital diagram for CN. Note that the 1s orbitals are not shown. Identify the bond order of CN. O2 01 OOOOO 25- 0 2s Answer Bank The atomic orbitals on the left side of the molecular orbital diagram are those of The atomic orbitals on the right side of the molecular orbital diagram are those of. (Get Answer) - Based on the molecular orbital theory, (a ... Based on the molecular orbital theory, (a) Describe or copy and complete the MO energy diagram for O2+; (b) state the bond order; and (c) indicate its magnetism. (Molecular Orbitals for the n = 2 period, *indicate anti-bonding orbitals, you may use /or to represent electrons) oʻ2px *. 2py ,
Oxygen(O) electron configuration and orbital diagram Orbital Diagram for Oxygen (O) Oxide ion (O 2-) electron configuration Ground state electron configuration of oxygen is 1s 2 2s 2 2p x2 2p y1 2p z1. This electron configuration shows that the last shell of oxygen has six electrons. In this case, the valence electrons of oxygen are six. Explain the formation of O2 molecule using molecular ... The energy of σ 2 p z molecular orbital is greater than and molecular orbitals in nitrogen molecule. Write the complete sequence of energy levels in the increasing order of energy in the molecule. Compare the relative stability and the magnetic behavior of the following species: N 2 , N 2 + , N 2 − , N 2 2 + 40 f2- molecular orbital diagram The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital. (PDF) Accurate ab initio potential energy curve of O2. II ... The embedding CI space is that of 12 electrons in the full space of all 8 valence orbitals FORS12/8. Prediction of the six highest vibrational levels for the 3 g − ground electronic state of O 2 ...
What is the orbital diagram of oxygen? Of oxygen is 8. Atoms can either donate or receive electrons only in valence shell. 8 electrons in outermost shell is considered the most stable state of an atom as the shell will be fully occupied. The valency of oxygen is 2 as its electronic configuration is 2,6 and it need 2 electron to complete their octet.
Solved Complete the valence molecular-orbital diagram for ... Electronic configuration of Oxygen is So in O2 there is 12 valence e… View the full answer Transcribed image text : Complete the valence molecular-orbital diagram for oxygen, 0, 1,00 JT Žp 2p Answer Bank 02pm 21 Os
Question #84799 + Example - Socratic.org In order to draw oxygen's molecular orbital diagram, you need to start by taking a look at what atomic orbitals you have for an oxygen atom, #"O"#.. As you know, oxygen is located in period 2, group 16 of the periodic table and has an atomic number equal to #8#.This means that the electron configuration of a neutral oxygen atom must account for #8# electrons.
Special Case of Highly Electronegative Elements Atomic oxygen has 6 valence electrons and 4 valence orbitals (2s, 2p x, 2p y, and 2p z). We can draw a Lewis structure of molecular oxygen with a double bond between the oxygen atoms and 2 non-bonding pairs of electrons on each atom. However, experimentally we can determine that O 2 has 2 unpaired electrons. The Lewis structure seems to be ...
Solved complete this valence molecular orbital diagram for ... complete this valence molecular orbital diagram for oxygen O2 click the blue boxes to add electrons; Question: complete this valence molecular orbital diagram for oxygen O2 click the blue boxes to add electrons
Molecular Orbital (MO) Diagram of O2 - YouTube Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*...
molecular orbital diagram for o2 - penguinskiclub.org Penguin Ski Club of New Hampshire. Located in Lincoln NH near Loon Mountain. Menu and widgets
Construct the molecular orbital diagram fo... | Clutch Prep Construct the molecular orbital diagram for He 2 and then identify the bond order. Click within the blue boxes to add electrons. Bond order: a) 0. b) 0.5. c) 1. d) 1.5. e) 2. Learn this topic by watching MO Theory: Bond Order Concept Videos.
3.3.4: Assembling a complete MO diagram - Chemistry LibreTexts Exercise 3.3.4. 3. Construct a qualitative molecular orbital diagram for chlorine, Cl 2. Compare the bond order to that seen in the Lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). Answer.
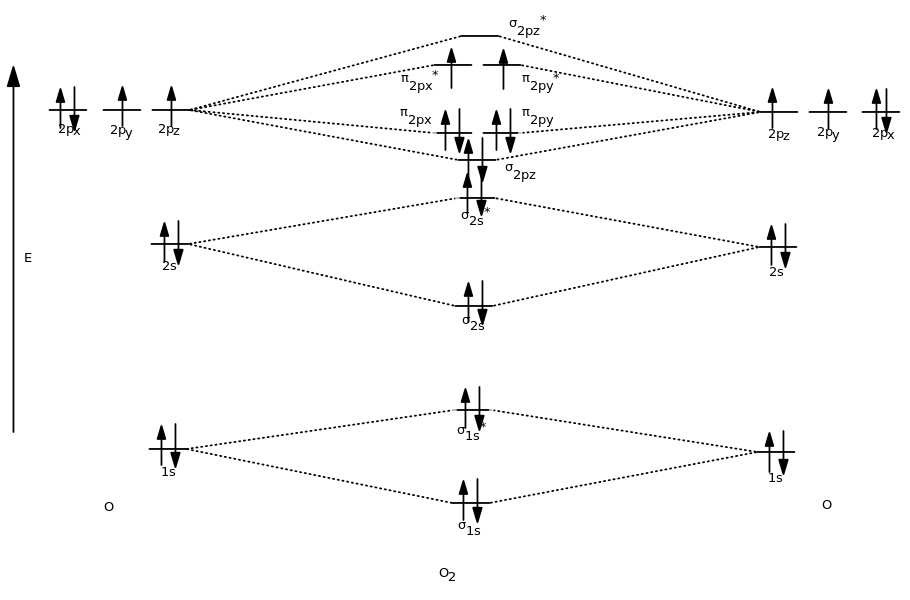

0 Response to "43 complete this valence molecular-orbital diagram for oxygen o2"
Post a Comment