42 the cell diagram for the lead-acid cell that is used in automobile and truck batteries is
A well cell battery generates power from a pair of electrodes and a liquid electrolyte solution. These are comprised of lead plates in a solution of sulfuric acid, hence referred as lead acid batteries also, and are commercially used for over 100 years. Related: The Correct Way to Jump-Start a Car Battery. Wet cell batteries are commonly ... The cell diagram for the lead-acid cell that is used in automobile and truck batteries is. The comma between PbO2 (s) and )PbSO4 (s) denotes a heterogeneous mixture of the two solids. The right-hand lead electrode is nonreactive. Write the balanced equation for the net cell reaction. Look up standard potentials for the oxidation and reduction ...
The lead acid battery in your automobile consists of six cells connected in series to give 12 V. Their low cost and high current output makes these excellent candidates for providing power for automobile starter motors. Visit this site for more information about lead acid batteries. Fuel Cells
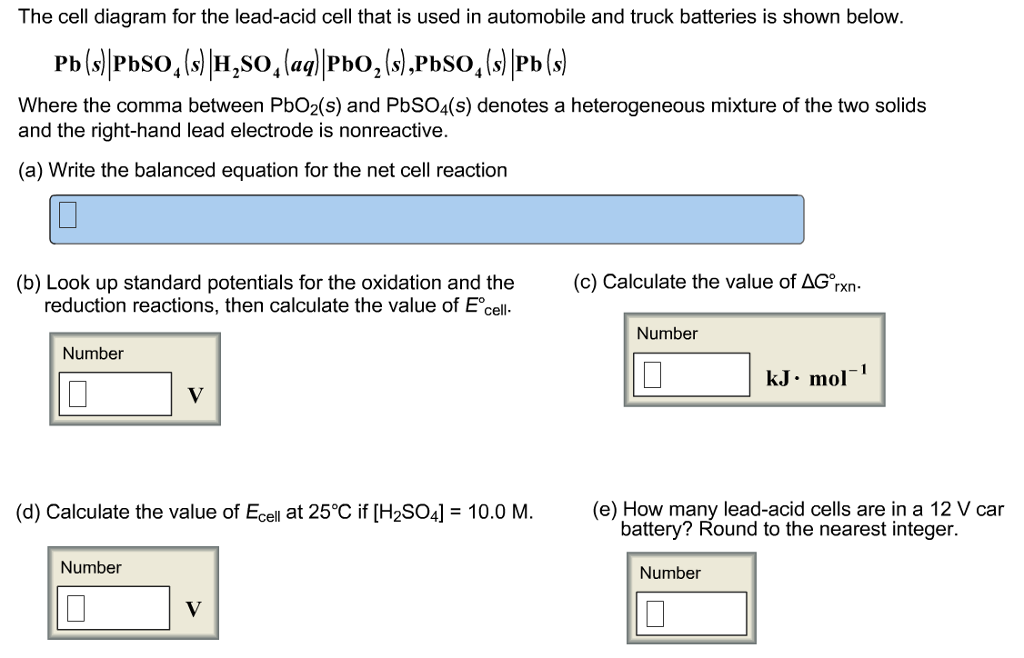
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb (s) l PbSO4 (s) l H2SO4 (aq) l PbO2 (s), PbSO4 (s) l Pb (s) where the comma between PbO2 (s) and PbSO4 (s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive. 25.10.2021 · The differences with Li-ion lie in a higher voltage per cell, tighter voltage tolerances and the absence of trickle or float charge at full charge. While lead acid offers some flexibility in terms of voltage cut off, manufacturers of Li-ion cells are very strict on the correct setting because Li-ion cannot accept overcharge. The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb(s)|PbSO4(s)|H2SO4(aq)|PbO2(s), PbSO4(s)|Pb(s) where the comma between PbO2(s) and PbSO4(s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive. (a) Determine the equation for the net cell reaction.
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is. Write the balanced equation for the net cell reaction. equation: Look up standard; Question: The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb(s)||PbSO4(s)||H2SO4(aq)||PbO2(s),PbSO4(s)||Pb(s) The comma between PbO2(s) and PbSO4(s) denotes a heterogeneous mixture of the two solids. The right-hand lead ... The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb (s)|PbSO4 (s)|H2SO4 (aq)|PbO2 (s),PbSO4 (s)|Pb (s) The comma between PbO2 (s) and PbSO4 (s) denotes a heterogeneous mixture of the two solids. The right-hand lead electrode is nonreactive. The cell diagram for the lead-acid cell that is used in automobile and truck batteries isWhere the comma between PbO2 (s) and pbSO4 (s) denotes a heterogeneous mixture of the two solids and the right ?hand lead electrode is nonreactive. The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb(s)|PbSO4(s)H, SO, (aq)|P60, (8), PbSO4) Pb(s) The comma between PbO,(s) and PbSO (3) denotes a heterogeneous mixture of the two solids. The right-hand lead electrode is nonreactive.
N. Maleschitz, in Lead-Acid Batteries for Future Automobiles, 2017 11.6 Outlook for the lead-acid design for further advanced high-rate applications. The lead-acid battery industry is often challenged about its future. Many people have stated that there is no future for the lead-acid battery, but there is nothing new about this, and despite decades of predictions about the demise of the ... The cell diagram for the lead-acid cell that is used in automobile and truck batteries is. Pb(s)||PbSO4(s)||H2SO4(aq)||PbO2(s),PbSO4(s)||Pb(s)Pb(s)|PbSO4(s)|H2SO4(aq)|PbO2(s),PbSO4(s)|Pb(s) The comma between PbO2(s)PbO2(s) and PbSO4(s)PbSO4(s) denotes a heterogeneous mixture of the two solids. The right-hand lead electrode is nonreactive. Hi! We notice you're using an ad blocker. Please consider allowing Autoblog. We get it. Ads can be annoying. But ads are also how we keep the garage … The cell diagram for the lead-acid cell that is used in automobile and truck batteries is shown below Where the comma between Pbo2(s) and Pbso4(s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive.
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb(s)∣∣PbSO4(s)∣∣H2SO4(aq)∣∣PbO2(s),PbSO4(s)∣∣Pb(s) The comma between PbO2(s) and PbSO4(s) denotes a heterogeneous mixture of the two solids. The right-hand lead electrode is nonreactive. Write the balanced equation for the net cell reaction. 12 -4: Lead -Acid Wet Cell This cell is a widely applied type of secondary cell, used extensively in vehicles and other applications requiring high values of load current. The positive electrode is made of lead peroxide. The negative electrode is made of spongy lead metal. The electrolyte is sulfuric acid. The output is about 2.1 volts per cell. The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb (s) l PbSO 4 (s) l H 2 SO 4 (aq) l PbO 2 (s), PbSO 4 (s) l Pb (s) where the comma between PbO 2 (s) and PbSO 4 (s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive. Each cell produces 2 V, so six cells are connected in series to produce a 12-V car battery. Lead acid batteries are heavy and contain a caustic liquid electrolyte, but are often still the battery of choice because of their high current density. Since these batteries contain a significant amount of lead, they must always be disposed of properly.
25.10.2021 · Lead acid batteries are generally charged till the voltage reaches 13.8V at 25ºC (more at colder, less at hotter temperatures) The rate of charge is generally limited at about 1/10 the Ah rating of the battery. Each manufacturer has their specs, depending on the application. … charging volts mean for the lifespan of the battery…
2. Vented Lead Acid Batteries 2.1 Hazards Vented lead acid batteries are commonly called "flooded", "spillable" or "wet cell" batteries because of their conspicuous use of liquid electrolyte (Figure 2). These batteries have a negative and a positive terminal on their top or sides along with vent caps on their top.
Because they are relatively cheap and easy to recharge, lead-acid batteries are useful for automobiles. A disadvantage of the lead-acid cell is that the electrolyte may leak or spill. Fuel cell: an open cell that requires the continuous addition of fuel-----Fuel cells are like batteries, however they do not run down or require recharging.
Concentration cell based on the Ni 2+ -Ni cell reaction. (a) Concentrations of Ni2+ (aq) in the two half-cells are unequal, and the cell generates an electrical current and a voltage. (b) The cell operates until [ Ni2+] is the same in the two half-cells, at which point the cell has reached equilibrium and the emf goes to zero.
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb(s)|PbS04(s)|H2S 0 4(aq)|Pb02(s),PbS04(s)|Pb(s) where the comma between PbO2 (s) and PbSO4 (s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive.
The lead-acid battery is a type of rechargeable battery first invented in 1859 by French physicist Gaston Planté.It is the first type of rechargeable battery ever created. Compared to modern rechargeable batteries, lead-acid batteries have relatively low energy density.Despite this, their ability to supply high surge currents means that the cells have a relatively large power-to-weight ratio.
Wet Cell; Valve-Regulated Lead-Acid. Here's a look at both categories and their respective sub-groups. Wet Cell / Flooded Batteries. These are the old school lead-acid batteries we've been using for years. Probably your parents and grandparents used them too. Within this category, there are two main types of battery, plus a hybrid style. 1.
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb (s) l PbSO4 (s) l H2SO4 (aq) l PbO2 (s), PbSO4 (s) l Pb (s) where the comma between PbO2 (s) and PbSO4 (s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive.
The cell diagram for the lead-acid cell that is used in automobile and truck batteries isPb(s) l PbSO4 (s) l H2SO4 (aq) l PbO2(s), PbSO4(s) l Pb... Q. Concentration cell based on the Ni2+-Ni cell reaction.
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb (s) l PbSO4 (s) l H2SO4 (aq) l PbO2 (s), PbSO4 (s) l Pb (s) where the comma between PbO2 (s) and PbSO4 (s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive.
For the GC/GM Impreza WRX, the EJ20G engine used a hot-film type mass air flow sensor to calculate intake air volume. The injection and firing order for the EJ20G engine was 1-3-2-4. The EJ20G engine had centrally mounted spark plugs and a compression ratio of 8.0:1.
Most trucks rely on two lead-acid batteries to do the cranking. When in good condition, this type of battery does an excellent job. But, there also are a number of drawbacks to using this type of ...
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb(s)|PbSO4(s)|H2SO4(aq)|PbO2(s), PbSO4(s)|Pb(s) where the comma between PbO2(s) and PbSO4(s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive. (a) Determine the equation for the net cell reaction.
25.10.2021 · The differences with Li-ion lie in a higher voltage per cell, tighter voltage tolerances and the absence of trickle or float charge at full charge. While lead acid offers some flexibility in terms of voltage cut off, manufacturers of Li-ion cells are very strict on the correct setting because Li-ion cannot accept overcharge.
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb (s) l PbSO4 (s) l H2SO4 (aq) l PbO2 (s), PbSO4 (s) l Pb (s) where the comma between PbO2 (s) and PbSO4 (s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive.


0 Response to "42 the cell diagram for the lead-acid cell that is used in automobile and truck batteries is"
Post a Comment