45 co3+ orbital diagram
The σ orbitals (black in the Energy Level Diagram) lie symmetrically across the π nodes of the πx or πy orbitals (red), so σ and π MOs do not mix ClO3- Lewis Structure, Molecular Geometry, Hybridization & Shape. May 3, 2021. Posted by Priyanka. 17 Apr. The chemical formula ClO 3- represents Chlorate ion. Chlorine can reach oxidation states of +1, +3, +5 and +7. In this case, as seen in the figure, Chlorates exist in a +5 oxidation state. With an abundance of oxidizing elements, the ...
co2 regulator parts diagram; co3+ orbital diagram; coleman rv air conditioner wiring diagram; columbine library diagram; communism vs socialism venn diagram; company er diagram; complete an orbital diagram for boron. confucianism and taoism venn diagram; conifer life cycle diagram; copper electron dot diagram; craftsman lt1000 deck belt diagram

Co3+ orbital diagram
All groups and messages ... ... These equivalent structures are called RESONANCE STRUCTURES. Three equivalent structures for the carbonate ion – all have the same formal charge. C O These equivalent structures are called RESONANCE STRUCTURES. The double bond electron pair is DELOCALIZED over all three C-O bonds and hence ... What Is The Hybridization Of Carbon In Co32−? April 26, 2021 by Admin. One unhybridized 2p orbital of carbon will laterally overlap with one unhybridized 2p orbital of oxygen to form pi-bond. Thus, there is a presence of one double bond in the carbonate ion. - Therefore, the carbon atom in carbonate ion, C O2 − 3 is sp2 hybridized.
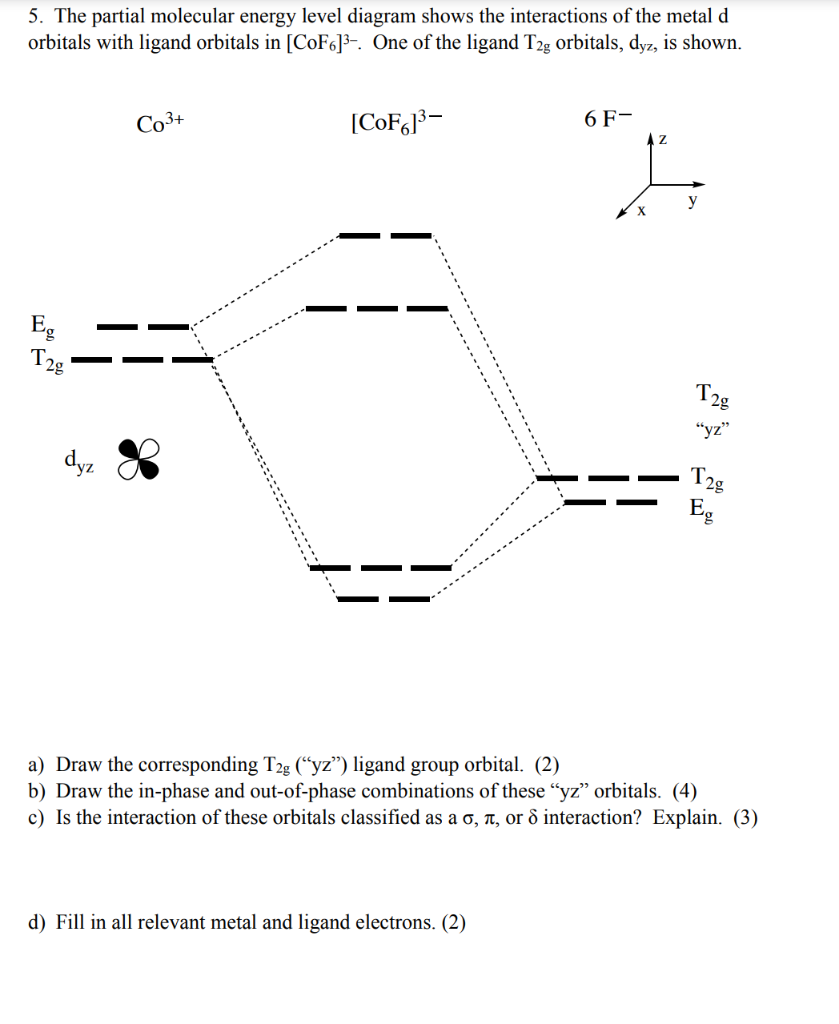
Co3+ orbital diagram. S.Chand Textbook of Chemistry Sem-I H.P.Shimla σ z y x σ* x y z Construct the molecular orbital diagram for Carbonate Ion is a polyatomic ion with formula of CO3 (2-). The system minimizes the need for moving mechanical components and is comprised of two subsystems that can be containerized for compact transportation. Jan 03, 2016 · The electron configuration of Co3+ is [Ar]4s3d5. Co is in Period 4 of the Periodic Table, and Ar is the preceding noble gas. Cobalt is also in Group 9, so it must have 9 valence electrons. The valence shell configuration is therefore 4s23d7, and the core notation is. When a transition metal forms an ion, the s electrons are removed before the d ... O. diagram for [Co(NH3)6]3+ Dr. Mithil Fal Desai Shree Mallikarjun and Shri Chetan Manju Desai College Canacona Goa 2. t* 1u a1g t2g, eg a1g, t1u, eg a1g t1u a* 1g e* g eg t1u Δo t2g Metal (Ti3+)orbitals Co3+→[Ar] 3d6, 4s0 6e- Ligand group (NH3) orbitals 6 x 2 = 12 e- σ [Co(NH3)6]3+ molecular orbitals M. O. diagram for [Co(NH3)6]3+ complex ...
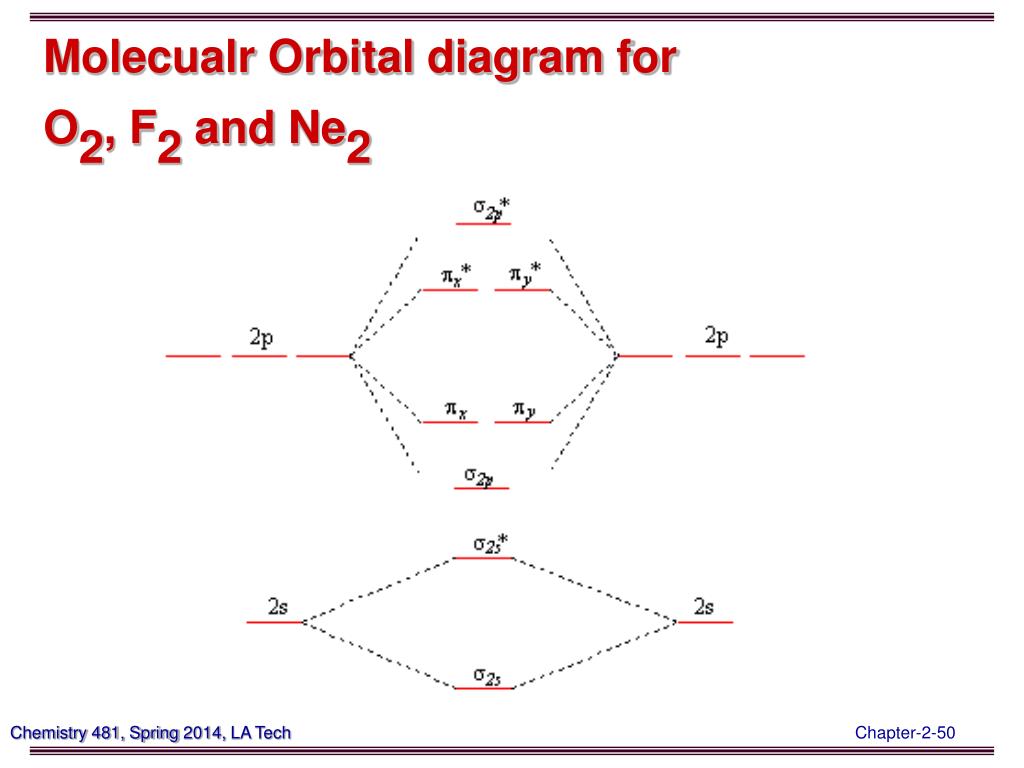
Cobalt (Co) has an atomic mass of 27. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. A simple notation used to represent valence electrons in an atom is called Lewis symbol. According to him, atoms achieve stable octet by gaining, loosing or ... A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that ... In molecular orbital (MO) approach - overlap orbitals for the whole ... Thus we can draw ENERGY LEVEL DIAGRAM for m.o.'s of H2 ... Carbonate ion, CO3.
September 13, 2020 - Problem CO3.2. Draw the frontier-orbital diagram (including orbital pictures) for the following compounds. This textbook has been designed to meet the needs of B.Sc. First Semester students of Chemistry as per the new UGC Model Curriculum - Choice Based Credit System (CBCS). With its traditional approach to the subject, this textbook lucidly explains principles of chemistry. When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i... 12-12 This video describes the molecular orbital theory diagram of CO, placing emphasis on how MO theory differs for homo and heteronuclear diatomics
SO42- Lewis Structure, Hybridization, Bond Angle and Molecular Geometry. SO42- is a chemical name of the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms and a charge of -2. It is a polyatomic anion and is used widely to synthesize other sulfates such as Zinc Sulfates, Magnesium sulfates, Iron sulfates and much more.
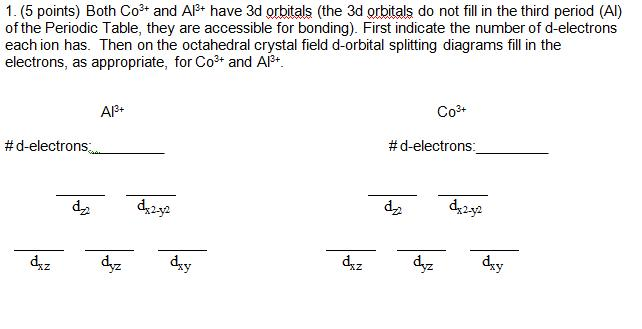
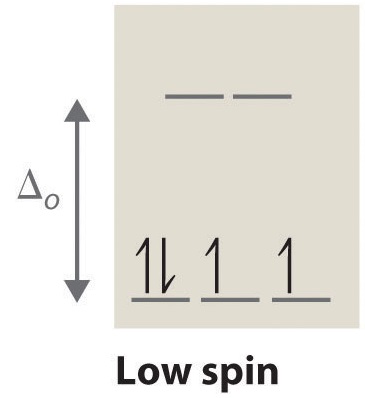
Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount of 0.6 Δo The t2gorbitals to be stabilized to the ...
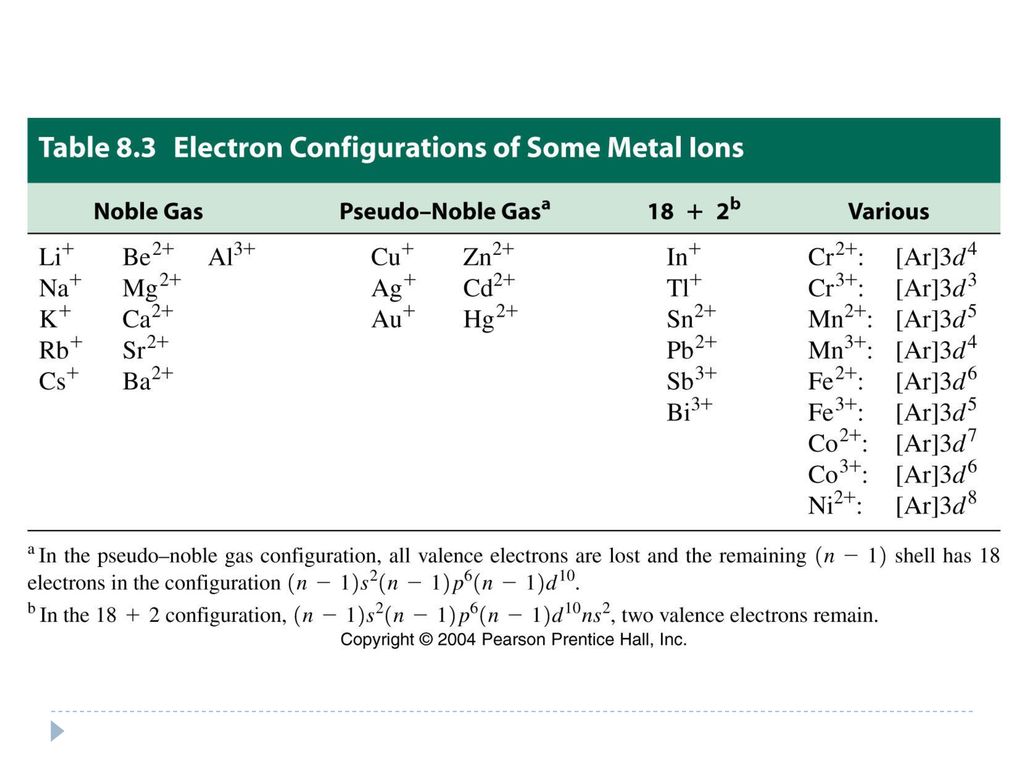
June 6, 2019 - electron configuration for co3+ ... configuration of co2+ cobalt iii ion electron configuration cobalt ii ion electron configuration cobalt iii ion co3+ orbital diagram which element forms a 3+ ion that has the electron configuration [kr]4d6 ? co3+ electron configuration ground ...
Carbonate Ion is a polyatomic ion with formula of CO3 (2-). Carbonate is a carbon oxoanion. It is a conjugate base of a hydrogencarbonate. Salts or ions of the theoretical carbonic acid, containing the radical CO2 (3-). Carbonates are readily decomposed by acids.
39 which model is most useful in developing a state machine diagram. State Machine Model with Auto-Billing Features. Finite State Machine (FSM) modelling is the most crucial part in developing proposed model as this reduces the hardware. In this paper the process of four state (user Selection, Waiting for money insertion, product delivery and ...
January 28, 2017 - Molecular orbital : Stability of molecule is determined by bond order.Higher is the Bond order greater is the stability of molecule.
What is the hybridization of carbon in CO3? One unhybridized 2p orbital of carbon will laterally overlap with one unhybridized 2p orbital of oxygen to form pi-bond. Thus, there is a presence of one double bond in the carbonate ion. - Therefore, the carbon atom in carbonate ion, CO2−3 is sp2 hybridized.
Co +3= 3d6 CN- is considered as strong ligand, hence all 6 electrons will be filled in t2g orbitals. All paired. Diamagnetic, inner orbital complex, d2sp3. Now in case of Br- is considered as weak ligand hence all 6 electrons will be filled in t2g and eg orbitals. ie., ( t2g)4, ( eg)2.
January 14, 2018 - Something went wrong. Wait a moment and try again
Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
circulation diagram architecture; co3+ orbital diagram; coleman rv air conditioner wiring diagram; columbine library diagram; confucianism and taoism venn diagram; craftsman lt1000 deck belt diagram; diagram of covalent bond; diagram of throat; dna replication fork diagram labeled; driver diagram templates; easy heart diagram; echo trimmer ...
11/01/2022 · Co3+ orbital diagram. Transcribed image text: Draw an orbital diagram for the Zn2+, Cu2+, Co2+, Fe2+, Fe3+, and Cr3+ ions in the presence of solvent molecules. Use up and down arrows to represent the spin of electrons. Only two electrons can occupy a single box. Fill the lower three boxes before filling the upper two and make sure to follow Hund's rule as you …
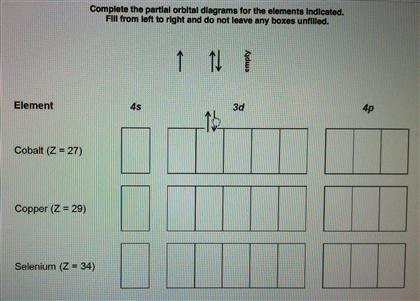
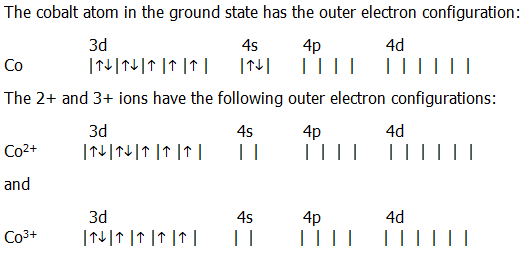
Atomic Orbital Diagram for Cobalt (Co) Cobalt ion(Co 2+,Co 3+)electron configuration. Ground state electron configuration of cobalt(Co) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 7 4s 2. The electron configuration shows that the last shell of cobalt has two electrons and the d-orbital has a total of seven electrons. In this case, the valence electrons of cobalt are nine. There are two types of cobalt ions.
Bond order=1/2 (bonding−anti-bonding) According to molecular orbital diagram, the bond order of CO+ is 3.5. The highest occupied molecular orbital is sigma*2s MO. In the case oc CO, the 2s atomic orbital on oxygen is much lower than the energy than the 2s atomic orbital of carbon. This discrepancy of energy allows the pi2px & pi2py BMO to ...
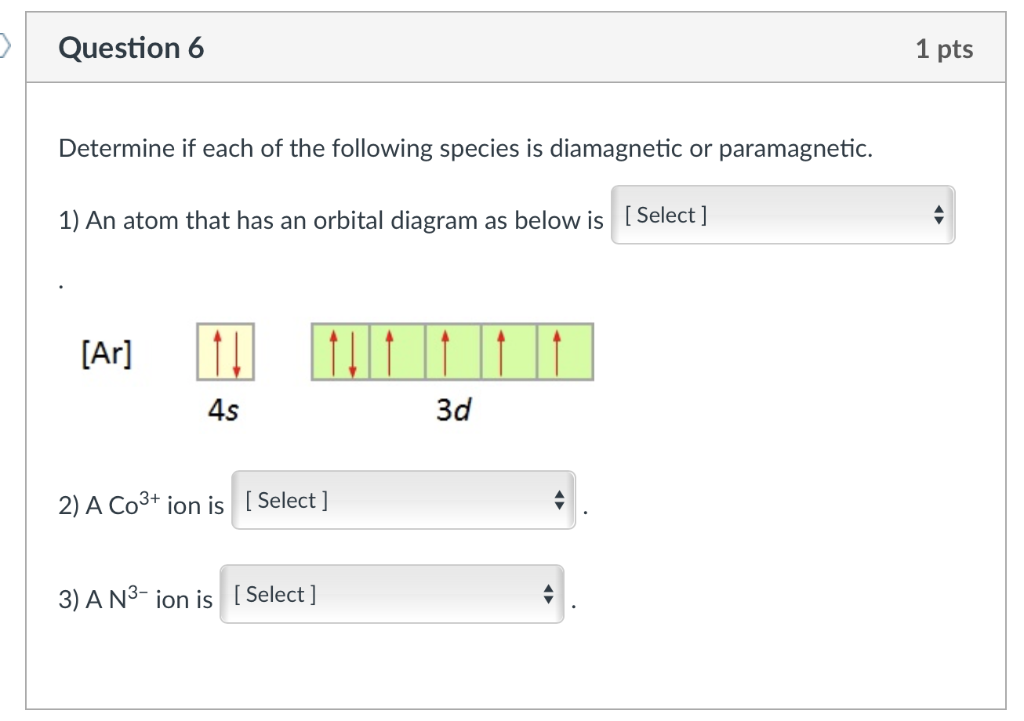
By Hund's rule, the electron configuration of carbon, which is 1s2 2s2 2p2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Which of the following has maximum number of unpaired electrons fe3+ Fe2+ Co2+ Co3 ...
Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. ... Answer to: Write the ground state electron configuration for Co2+ and Co3+. Sodium 1s^2 2s^2 2p^6 3s^1 2. This is the reason that bismuth is in period six.
Note that the bonding orbital in the MO diagram of H2 is stabilized by an energy β/1+S and the antibonding orbital is destabilized by β/1-S. That is, the antibonding orbital goes up in energy more than the bonding orbital goes down. This means that H2 (ψ12ψ20) is energetically more stable ...
July 23, 2018 - Download scientific diagram | Valence bond structure of carbonate ion (CO3 2-). from publication: Review of Innovative Mnemonics for Inorganic and Organic Chemical Education | In this review article, formulae based innovative mnemonics have been discussed to create interest and remove phobia ...
New bifunctional TCNQ-based material · New bifunctional TCNQ based material: [Co(terpy)2](TCNQ)3·CH3CN exhibits a high room temperature conductivity of 0.13 S cm−1 and an anomaly in conductivity at ∼190 K as evidenced by variable temperature structural, magnetic and conductivity studies
To write the configuration for the Cobalt ions, first we need to write the electron configuration for just Cobalt (Co). We first need to find the number of ...
An electron from the s orbital will transfer to the d orbital. A good example is chromium (CR): ... Cr2+,Cu2+,Co3+ Write out the ground-state electron configurations of the following ions. A.) Ti ...
Start studying Mitosis and Meiosis Venn diagram. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Mitosis vs. Meiosis Venn Diagram Compare and Contrast Mitosis and Meiosis ID: 1408281 Language: English School subject: Biology Grade/level: 9 Age: 14-15 Main content: Science Other contents: Add to my workbooks (40) Download file pdf Embed in my website or blog Add ...
How many electrons does Cobalt Co have in the 3d sublevel? 7 electrons. The electronic configuration of cobalt is $ [Ar]3 {d^7}4 {s^2} $ . Electrons present in the outermost electronic configuration of an atom are the valence electrons. 9 is the valence electron in the cobalt atom. 7 electrons are present in the $ 3d $ orbital and 2 electrons ...
SF6 Molecular Geometry, Lewis Structure, Shape, and Polarity. July 23, 2021. Posted by Priyanka. 16 Apr. Sulfur hexafluoride or SF6 is an inorganic, greenhouse gas. It is non-flammable, odourless, and colourless, and is an excellent insulator. It is a hypervalent octahedral molecule that has been an interesting topic of conversation among ...
Chemistry. Chemistry questions and answers. What is the molecular orbital diagram for a carbonate ion? CO3 2- Please include labels for the orbitals like T2g, pi, pi anti-bonding etc. Question: What is the molecular orbital diagram for a carbonate ion?
Electron Configuration Chart of All Elements (Full Chart) November 1, 2021 March 7, 2021 by Admin. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no.
April 5, 2014 - Answer (1 of 12): cobalt has the ground state electron configuration of [Ar] 4s2, 3d7 But when cobalt loses three electrons, it loses them from both the 4s and the 3d. It loses two electrons from the 3d in order to make the 3d half-filled, which has a high degree of stability, and it loses 1 elec...
A quick explanation of the molecular geometry of CO3 2- including a description of the CO3 2- bond angles.Looking at the CO3 2- Lewis structure we can see th...
In carbonate ion, molecular orbital theory can be best explained via delocalized pi bonding. A delocalized pi bond signifies that the electrons are free to have ...
LEWIS STRUCTURE- HYBRIDIZATION
The molecular orbital diagram of CO2 is as below. A molecular orbital diagram of any compound gives us an idea about the bonding of the orbitals. It also helps us to find the bond order, bond length, bond strength of the molecule. In the diagram, the left-hand side consists of the atomic orbitals of carbon.
What is the bond order of f22- according to molecular orbital theory? Refer to the diagram. the equilibrium price and quantity in this market will be; A hotel will use a job order cost system for which of the following expenses? Which of the following is an example of a postponement tactic? If the yield on a fixed coupon bond goes up
So so this is a orbital diagram of carbon in so to an indication of ... CO3 2- Please include labels for the orbitals like T2g, pi, pi anti-bonding etc.
Orbital explanation for the endo rule; Working out which product is endo; The Woodward Hoffman description of the Diels-Alder; Intramolecular Diels-Alder (E)-3-Methyldeca-1,3,9-triene; Intramolecular Diels-Alder – 1,3,9-decatrien-8-one; 2,3-Dimethylbutadiene and Acrolein(propenal) Quinone as Dienophile – Steroid Framework
Example showing how to draw orbital diagrams. Also Cobalt orbital diagram example problem.For more chemistry help videos and practice worksheets go to:https...
6 Predicting Electron Configurations of Ions What is the electron configuration and orbital diagram of: (a) Na + (b) P 3- (c) Al 2+ (d) Fe 2+ (e) Sm 3+ Solution First, write out the electron configuration … So, before writing the electronic configuration of an ion, you should first determine the total number of electrons with the ion.
May 20, 2016 - Answer (1 of 8): No. of valence electrons in central atom. (carbon)= 4 Extra electrons in CO3^-2 = 2 Total electrons in central atom = 6 Hybrid oribitals required = 6/2 = 3 Hybridization = sp2 Geometry = trigonal
Chemistry Q&A Library For the carbonate ion, CO3 2− 1- Draw the electron orbital diagram for the valence electrons of the central carbon before and after hybridization. 2- Identify which carbon and oxygen electron orbitals overlap to create each single and double C-O bond in the structure
What Is The Hybridization Of Carbon In Co32−? April 26, 2021 by Admin. One unhybridized 2p orbital of carbon will laterally overlap with one unhybridized 2p orbital of oxygen to form pi-bond. Thus, there is a presence of one double bond in the carbonate ion. - Therefore, the carbon atom in carbonate ion, C O2 − 3 is sp2 hybridized.
These equivalent structures are called RESONANCE STRUCTURES. Three equivalent structures for the carbonate ion – all have the same formal charge. C O These equivalent structures are called RESONANCE STRUCTURES. The double bond electron pair is DELOCALIZED over all three C-O bonds and hence ...
All groups and messages ... ...



















0 Response to "45 co3+ orbital diagram"
Post a Comment