43 phase diagram questions and answers
14 Questions Show answers Question 1 30 seconds Q. What state of matter is Y? answer choices solid liquid gas supercritical fluid Question 2 30 seconds Q. What state of matter is Z? answer choices solid liquid gas supercritical fluid Question 3 30 seconds Q. What change occurs from F to E? answer choices sublimation deposition melting condensation The following questions require some thought and reaching the answer may require you to think beyond the contents of this TLP. Using the following data, calculate the volume fraction of the beta phase and eutectic at the eutectic temperature, for an alloy of composition 75 wt% Ag.
Clarification: A unary phase diagram has a single component thus, at triple point, the number of degrees of freedom, according to Gibbs phase rule F = 1 - 3 + 2 = 0. 3. The invariant reaction involving, a liquid phase decomposing into two different solids on cooling is known as _________
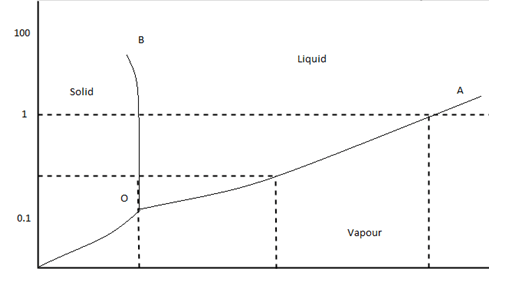
Phase diagram questions and answers
1. To measure freezing point depression the following equation is used Delta T = - i Kf m. What is the m in this equation? molarity moles molar mass mass molality 2. In the broadest sense, what was... Phase diagram questions. 14:440:407 ch9 Question 9.1 Consider the sugar–water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90°C (194°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid. What will be the composition of the ... Answer the following questions based on this phase diagram. (Note: The phase diagram is made-up by the instructor, and does not represent an actual substance.) Show all questions <= => The area of the graph that represents the solid phase is: ? A ?
Phase diagram questions and answers. One component diagrams C=1 therefore F=C-P+2=3-P One component diagrams Detection of phase transitions and building a phase diagram is based on calorimetry measurements Two-components diagrams C=2 therefore F=4-P. We have to reduce degree of freedom e.g. by fixing T=const •Vapour pressure diagrams p A==xp p xp AA B B B Raoult's Law A phase diagram is a graphical way to depict the effects of pressure and temperature on the phase of a substance: ... (NOTE: multiple answers needed for this question) 22) If I had a quantity of this substance at a pressure of 2.00 atm and a temperature of -1500 C, what phase change(s) would occur if I decreased the pressure to 0.25 atm? At ... See what you know about phase diagrams and what they're used for. The practice questions on the quiz will test your understanding of the diagrams themselves, what one of these diagrams can and ... PHASE DIAGRAM WORKSHEET #2 Name_____ Period_____ Date_____ At standard temperature and pressure, bromine (Br 2) is a red liquid. Bromine sublimes when the temperature is -25 0C and the pressure is 101.3 kPa. The phase diagram for bromine is shown below. Use this diagram for questions (1) - (9)
2. Which of the following cannot be obtained using a phase diagram? a) Melting temperatures of various phases b) Temperature range for solidification c) Equilibrium solid solubility d) Purity of materials View Answer 3. A specific body of material or a series of alloys with the same compositions is/are known as _________ a) Component b) System You can read 35+ pages phase diagram questions and answers pdf solution in Google Sheet format. You will not be responsible for the new concepts that are somewhat incidental to this problem namely the microscope pictures in the circles in the diagram below and any new terminology such as eutectic structure and eutectic alpha Consider a 40 wt Sn-60 wt Pb alloy on the lead-tin phase diagram ... Materials Science and Engineering: An Introduction answers to Chapter 9 - Phase Diagrams - Questions and Problems - Page 349 9.4 including work step by step written by community members like you. Textbook Authors: Callister, William D.; Rethwisch, David G., ISBN-10: 1118324579, ISBN-13: 978-1-11832-457-8, Publisher: Wiley asked a question related to Phase Diagrams Can anyone help me with what I mention below? Question 1 answer Aug 13, 2021 I am looking for phase diagrams that help me in understanding how the salts...
158 questions with answers in PHASE DIAGRAMS | Scientific method Science topics: Engineering Materials Engineering Phase Diagrams Science method Phase Diagrams - Science method Explore the latest... The Cs-K phase diagram is given on the next page. Refer to it in answering the following questions. (a) For a sample of composition 20 at. % Cs and 80 wt. % K held at 10°C, determine (i) the composition of the solid phase present at equilibrium (ii) the composition of the liquid phase present at equilibrium Iron carbon phase diagram questions and answers pdf Fig 1: Fe-Fe3C Phase Diagram (clickable), Materials Science and Metallurgy, 4th ed., Pollack, Prentice-Hall, 1988 Figure 1 shows the equilibrium diagram for combinations of carbon in a solid solution of iron. The diagram shows iron and carbons combined to form Fe-Fe3C at the 6.67%C end of the Phase Diagrams: composition of phases At TA= 1320°C: Only Liquid (L) present CL= C0 ( = 35 wt% Ni) At TB= 1250°C: Both and L present At TD= 1190°C: Only Solid ( ) present C = C0( = 35 wt% Ni) C L = C liquidus ( = 32 wt% Ni) C = C solidus ( = 43 wt% Ni) 18 • Rule 3:If we know T and Co, then we know: --the amount of each phase (given in wt%).
H3.3.1 Building up a binary phase diagam A simple introduction Webeginwithafamiliarexampletoestablishsomefundamentalbehavior.Considerthetwo-component system: water and salt. Each is a compound with a definite chemical composition, so we treat their mixturesasconsistingoftwocomponents,ratherthanasfourelements.
Part D – Phase Diagram for Tastegudum. On Crosbia, bolonium (Bg) and manasium (Ma) react together to form the compound tastegudum. For each of the following questions (16-28), refer to the phase diagram for tastegudum. See Miss Scott for answer key with labels. Label the regions of the diagram that correspond to the solid, liquid, and vapor ...
Use the graph to answer the following questions. A phase diagram is a graphical way to depict the effects of pressure and temperature on the phase of a substance. Heating Curves Biology Worksheet Quizzes And Answers Chemistry 175 150 125 075 050 025 000 Temperature degrees C 2 3 4 6 Label the following on […]
Phase Diagram Worksheet Answers Refer to the phase diagram below when answering the questions on this worksheet: 1.75 1.50 1.25 0.75 0.50 0.25 0.00 Temperature {degrees C) 2) 3) 4) 6) Label the following on the phase diagram above: Solid phase, liquid phase, gas phase, triple point, critical point.
Using a phase diagram, one can obtain at least the following three information. 1. The phases that are present, 2. The composition of each phase, and 3. The amount of each phase present. 17. What is tie-line?
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
Transformer MCQ Questions and Answers PDF. 1. The primary and secondary windings of a power transformer always have. (a) a common magnetic circuit. (b) separate magnetic circuits. (c) wire of same size. (d) same number of turns. Answer: (a) a common magnetic circuit. 2.
Q. Water exists as a _____________ at 700 mmHg and 50 °C. Q. What phase change (s) may occur at pressures below 4.58 mmHg? Q. The pressure is increased on a sample of water at 0 °C from 0 mmHg to 800 mmHg. In order, what changes occur? Q. Based on this phase diagram, which state is the most dense? Q. Based on its phase diagram, which is the ...
Question: 111 Using a phase diagram to predict phase at a given temperature... Study the following phase diagram of Substance X. 3.2 solid pressure (atm) 16 liquid gas 600 temperature (K) Use this diagram to answer the following questions.
Material Science/ Phase Diagrams. Multiple Choice Questions. Multiple Choice Questions: 1. Gibbs phase rule for general system: (a) P+F=C-1. (b) P+F=C+1.3 pages
Answers to Chemistry Problems Answers to Chemistry Problems; Chemistry Quiz Online Quizzes for CliffsNotes Chemistry QuickReview, 2nd Edition; Quiz: Phase Diagrams Previous Phase Diagrams. Next Heat Capacities and Transformations. Discovery and Similarity Quiz: Discovery and Similarity Atomic Masses ...
Answer the following questions based on this phase diagram. (Note: The phase diagram is made-up by the instructor, and does not represent an actual substance.) Show all questions <= => The area of the graph that represents the solid phase is: ? A ?
Phase diagram questions. 14:440:407 ch9 Question 9.1 Consider the sugar–water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90°C (194°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid. What will be the composition of the ...
1. To measure freezing point depression the following equation is used Delta T = - i Kf m. What is the m in this equation? molarity moles molar mass mass molality 2. In the broadest sense, what was...

0 Response to "43 phase diagram questions and answers"
Post a Comment