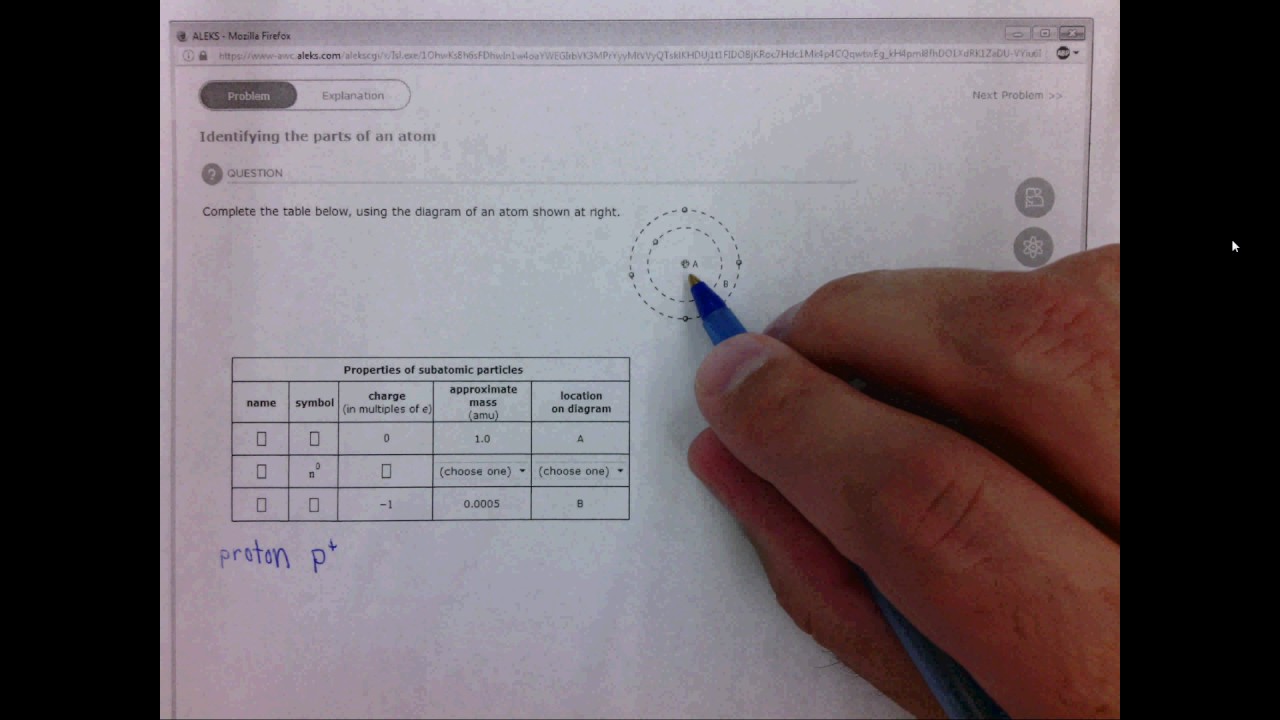
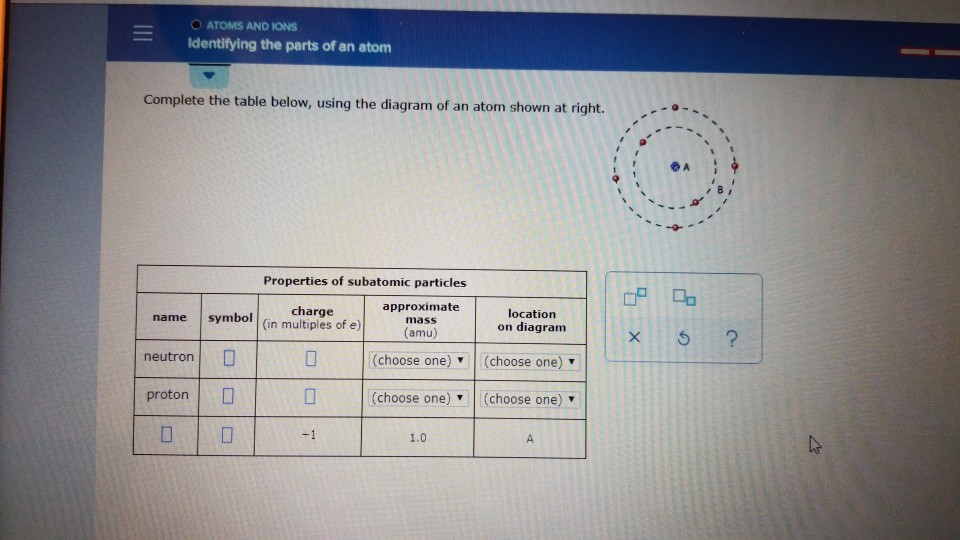
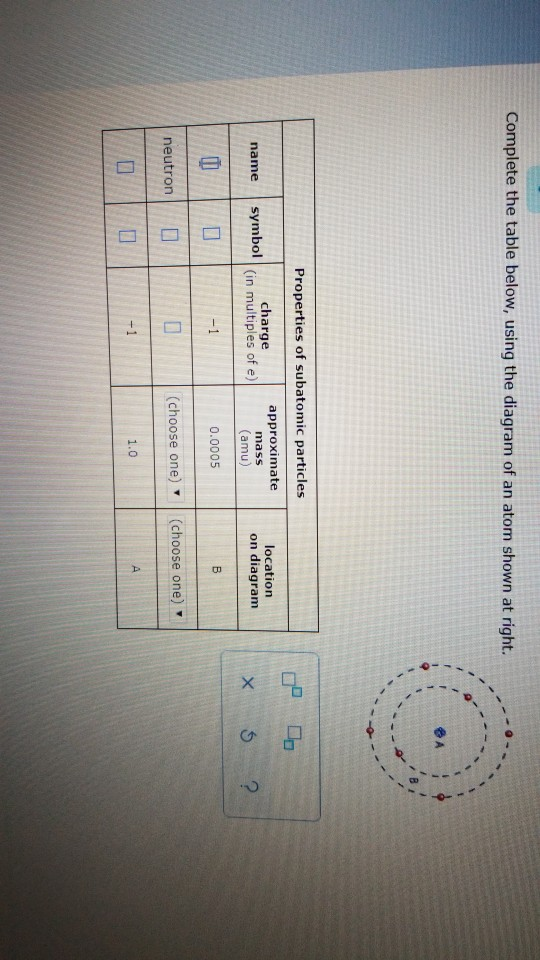
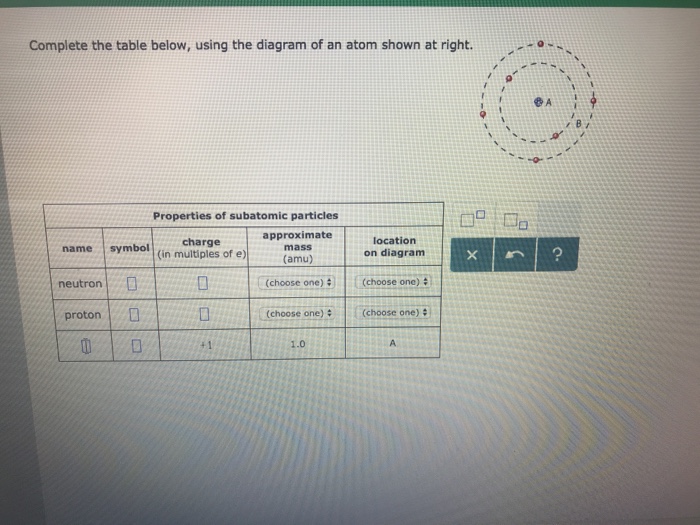
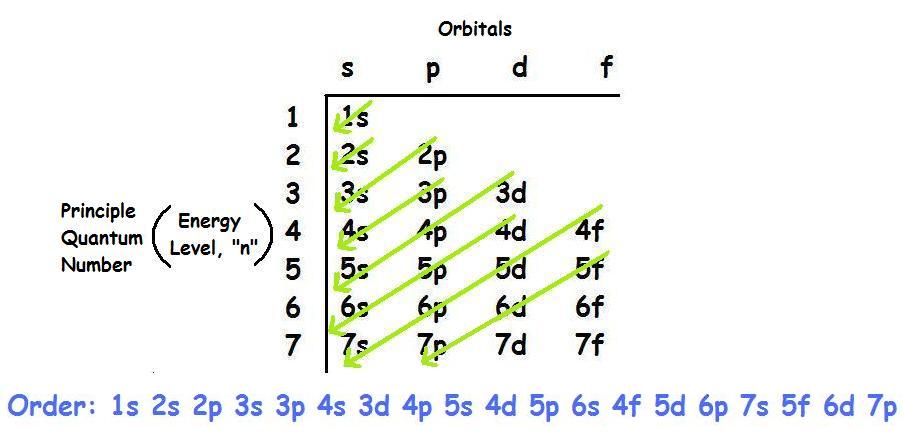
41 complete the table below, using the diagram of an atom shown at right.
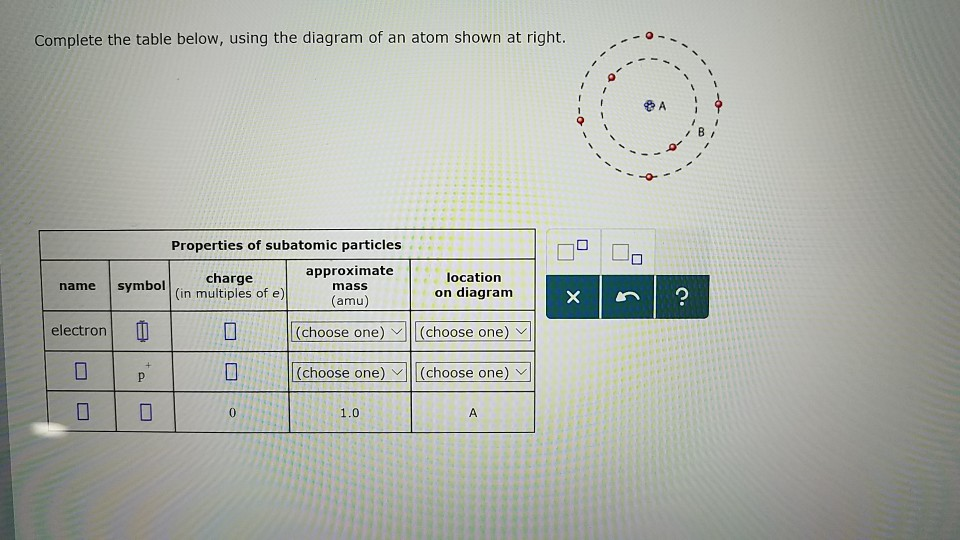
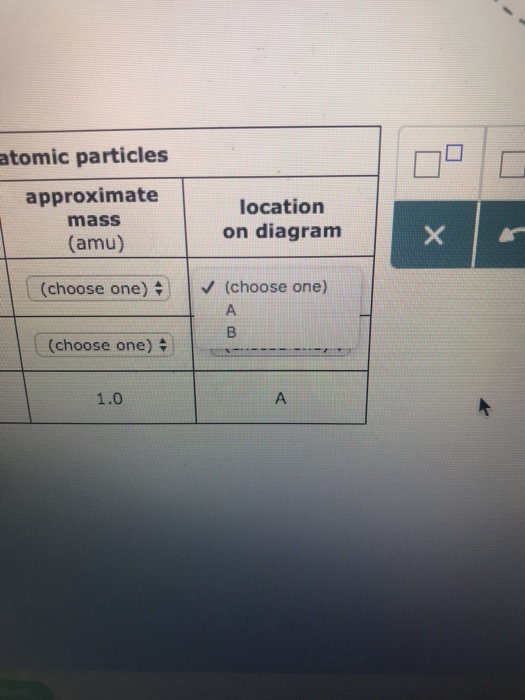
Transcribed image text: Complete the table below, using the diagram of an atom shown at right. Properties of subatomic particles approximate charge symbol ... Complete the table below, using the diagram of an atom shown at right. Properties of subatomic particles approximate symbol charge mass (in multiples of e) (amu) name location on diagram Х х 5 ? +1 1.0 А electron 0 (choose one) (choose one) 0 0.0005 B.
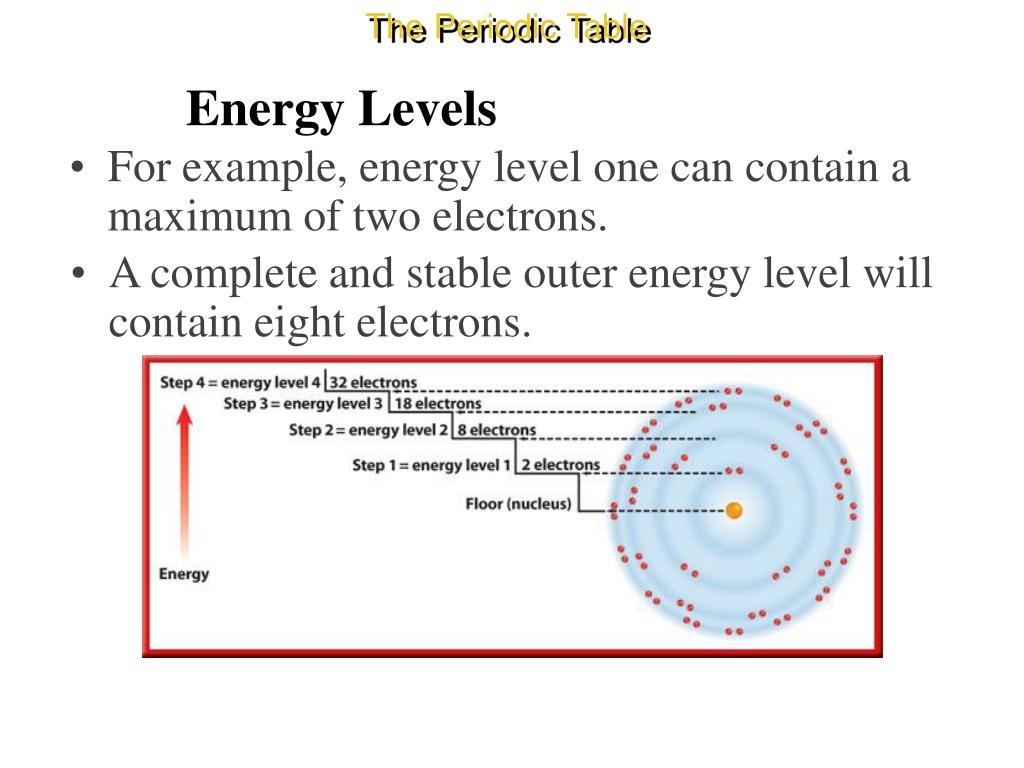
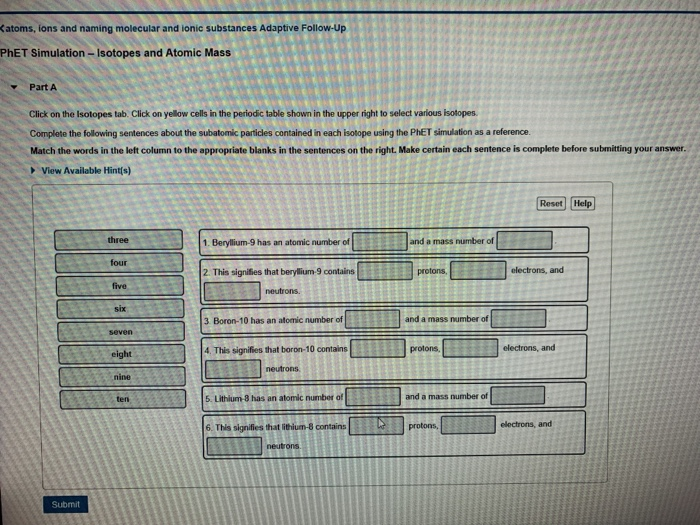
The atom shown in the diagram above has a total of 5 electron orbitals. ... Complete the sentences below by making use of the periodic table. Bromine has 35 protons. ... Atoms with an outer shell that is almost empty are located on the right side of the Periodic Table while atoms with an outer shell that is full or almost full are located on ...
Complete the table below, using the diagram of an atom shown at right.
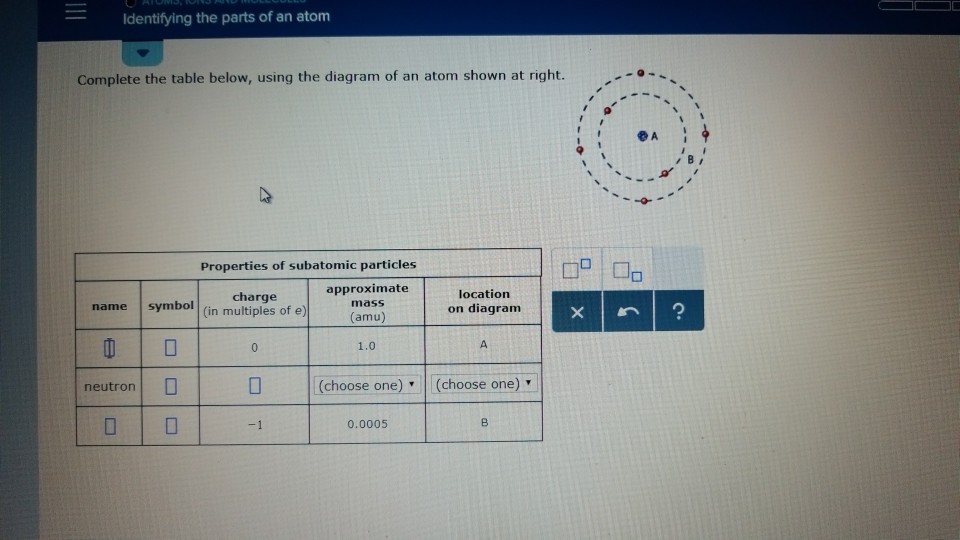
Question: Identifying the parts of an atom Complete the table below, using the diagram of an atom shown at right. Properties of subatomic particles charge ... Complete the table below, using the diagram of an atom shown at right. Properties of subatomic particles approximate location charge (in multiples of e) symbol mass on diagram name (amu) -1 0.0005 B 1.0, (choose one) (choose one) v :- Question: Complete the table below, using the diagram of an atom shown at right. This problem has been solved! See the answer ...
Complete the table below, using the diagram of an atom shown at right.. 2 in order to identifr the color of spectral lines produced in each of the hydrogen atom electron transitions shown in Model 3. Use colored pencils to trace the light wave in each of the four pictures with the appropriate color. 21. Consider the electron transitions in Model 3. a. Which of the electron transitions involves the most energy? b. (a) Complete the Lewis electron-dot diagram of methyl methanoate in the box below. Show all valence c— electrons. 5. Methanamide, CH3NO, is a liquid at 250C. H (a) The complete Lewis electron-dot diagram for methanamide is shown below. so is (i) In the molecule, angle x is not 1800. Estimate the observed angle. Justify your answer. FREE Expert Solution. To complete the table using the diagram: electrons →outside nucleus→(-) charge→ mass = 0.00549 amu. protons →inside nucleus→ (+) charge→mass = 1.007 amu. neutrons→inside nucleus →neutral charge→mass =1.008 amu. 92% (171 ratings) Sign up for free to view this solution. Sign up for free. 22, , as shown below. We will now repeat this process for a 60o reference angle. We first draw a right triangle that is based on a 60o reference angle, as shown below. We again want to find the values of x and y. The triangle is a 30o-60o-90o triangle. Since the length of the hypotenuse is 1 and it is twice the length of the shorter leg, x, we ...
Oct 17, 2021 · Complete the table below, using the diagram of an atom shown at right. A name Properties of subatomic particles Aharge approximate symbol mass (in multiples of e) (amu) name location on diagram X5 ? O 1.0 А 0 1.0 А proton (choose one) (choose one) Complete the table below, using the diagram of an atom shown at right. The box for an element from the periodic table is shown. Which is the atomic number. B. ... An atom of the element phosphorus (P) contains 15 electrons. Using the sub-level diagram below, determine which sub-level is partially filled. ... The difference between the atom on the left and the atom on the right is that the electron has been. Excited. Complete the table below, using the diagram of an atom shown at right. Properties of subatomic particles approximate charge |(in multiples of e) location name symbol mass on diagram (amu) ? +1 1.0 A p (choose one) (choose one) Drawing Dohr model diagrams 1. Refer to the Bohr model chart on page 32 to help you complete the following table. Some answers are provided for you. (Hint: Remember that the maximum number of electrons in the first three shells is 2, 8, and 8.) Number of electrons 10 10 10 14/ 18 18 Number of electron shells Atom/ion neon atom fluorine atom
(d) The Lewis electron-dot diagram for C2H4 is shown below in the box on the left. In the box on the right, complete the Lewis electron-dot diagram for C2H50H by drawing in all of the electron pairs. H ë:: H H ë H 1 point is earned for a correct diagram. Diagram should include all bonding pairs plus two nonbonding pairs on the O atom. 1 (a) Complete the diagrams to show the energies of the electrons in a carbon atom, a C+ ion and a C- ion. increasing energy 1s carbon atom ↑↓ C+ ion ↑↓ C- ion 1s 1s ↑↓ [2] (b) One of the simple molecular allotropes of carbon is buckminsterfullerene, C60. buckminsterfullerene (i) What is the hybridisation of the carbon atoms in C60? Complete the table below, using the diagram of an atom shown at right. Properties of subatomic particles approximate charge (in multiples of e) symbol location name mass (amu) on diagram +1 1.0 1.0 (choose one) v (choose one) v 4. Atoms tend to react in ways that give each atom a stable outer shell of electrons. true correct 5. Atoms with an outer shell that is almost empty are located on the right side of the Periodic Table while atoms with an outer shell that is full or almost full are located on the left side of the Periodic Table. false correct 6.
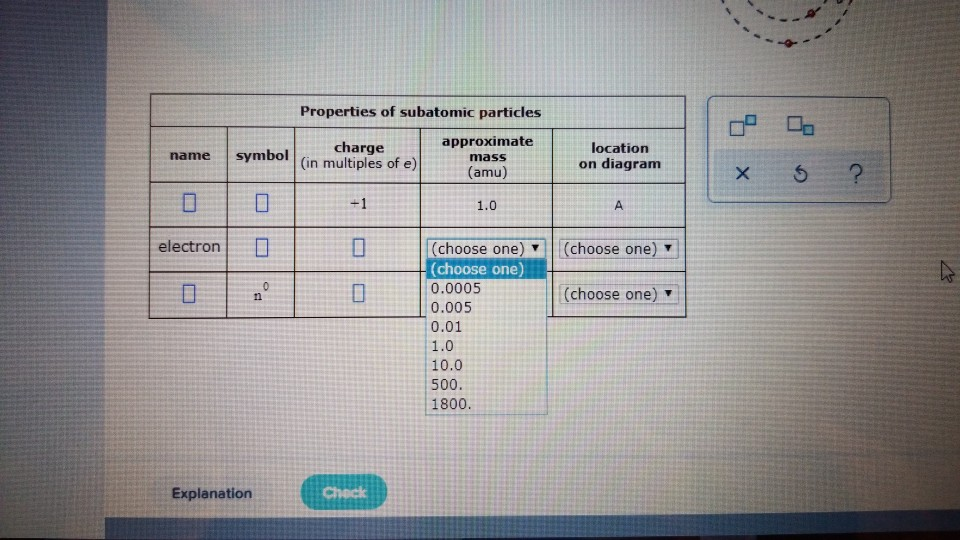
Chemistry questions and answers. Complete the table below, using the diagram of an atom shown at right. Properties of subatomic particles charge approximate symbol mass (in multiples of e) (amu) name location on diagram Х 0 0.0005 B P (choose one) (choose one) (choose one) 0.0005 0.005 0.01 10 10.0 500 1800 Explanation Check O ATOMS, IONS AND ...
Complete the table below, using the diagram of an atom shown at right. Properties of subatomic particles approximate charge (in multiples of e) location name symbol mass on diagram (amu) electron (choose one) (choose one) ♥ proton (choose one) v (choose one) ♥ -1 0.0005 B OOO
Chemistry. Chemistry questions and answers. Complete the table below, using the diagram of an atom shown at right. Do name Properties of subatomic particles charge approximate symbol mass (in multiples of e) (amu) 0 0 (choose one) location on diagram Х $ ? (choose one) D 0 +1 1.0 А 0 1.0 А.
Transcribed image text: Complete the table below, using the diagram of an atom shown at right. Properties of subatomic particles approximate mass (amu) ...
Complete the table below, using the diagram of an atom shown at right. -0- Properties of subatomic particles approximate name symbo (in multiples of e) location on diagram amu) (choose one) (choose one) 1.0 0 1.0
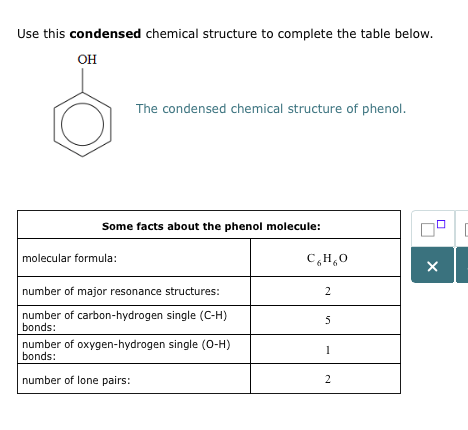
(c) A 'dot-and-cross' diagram of a CO molecule is shown below. Only electrons from outer shells are represented. In the table below, there are three copies of this structure. On the structures, draw a circle round a pair of electrons that is associated With each of the following. (i) a co-ordinate bond (ii) a covalent bond (iii) a lone pair
Transcribed image text: Complete the table below, using the diagram of an atom shown at right. Properties of subatomic particles charge approximate mass (in multiples of e) (amu) name symbol location on diagram X 5 ? +1 1.0 А neutron 0 (choose one) (choose one) 0 1.0 A Complete the table below, using the diagram of an atom shown at right.

Go to your garden, plant strawberries, wait. Wait. Wait. Well, wait few days, weeks, and enjoy your strawberries. This one is the first one from my garden.
Chemistry questions and answers. Complete the table below, using the diagram of an atom shown at right. name Properties of subatomic particles approximate symbol charge mass location (In multiples of e) on diagram (amu) р (choose one) (choose one) Х $ ? 0 0 0 n (choose one) (choose one) 0 +1 1.0.
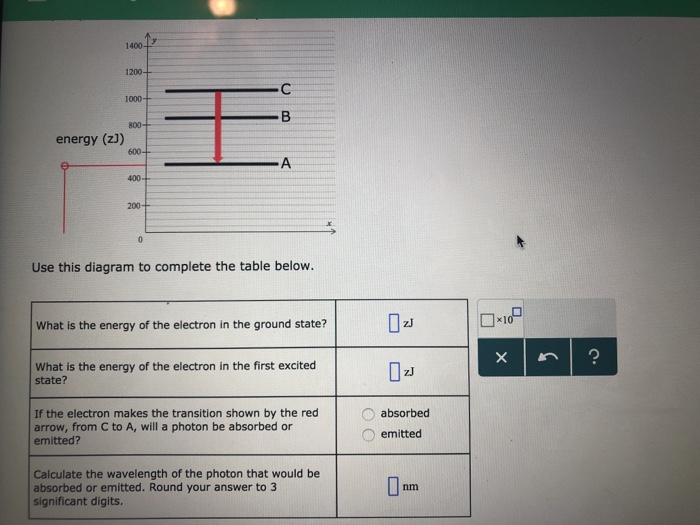
This reversible reaction is shown in equilibrium 3.1 below. H2 (g) + I2 (g) 2HI (g) ΔH = −9 kJ mol−1. The activation energy for the forward reaction is 173 kJ mol−1. (a) Complete the enthalpy profile diagram below for the forward reaction in equilibrium 3.1. On your diagram: • Label the activation energy, Ea.
Use your knowledge of atomic structure to label this diagram of a nitrogen atom. The boxes below are labeled with the approximate atomic masses of four different elements. Using the provided atomic masses and the periodic table shown, drag the appropriate subatomic particles into the boxes to create electrically neutral atoms.
Question: Complete the table below, using the diagram of an atom shown at right. This problem has been solved! See the answer ...
("Subatomic" means "smaller than an atom.") Here are the three types of subatomic particle you need to know for chemistry: name symbol charge (in multiples of e) mass (amu) location proton p + +1 1.0 nucleus neutron n 0 0 1.0 nucleus electron 1 0.0005 or about 1/1800 orbiting the nucleus Using these facts, which you must memorize, you can ...
the Periodic Table: Understanding the Main Ideas Pact Use different colored pencils to show each of the following items on the pcrtodtc table. Make a key to indicate which cotor are using for each item, group or family metals rtod nonmetals metalt ids Part 2 The diagram below shows a square from the periodic table. On the diagram,
(a)€€€€ (i)€€€€€€The electronic structure of a magnesium atom is shown below. Use the correct answer from the box to complete each sentence. € € electrons neutrons protons shells The nucleus contains protons and ..... The particles with the smallest relative mass that move around the nucleus are called
Complete ure table below, using the diagram of an atom shown at right. name Properties of subatomic particles charge approximate symbol in multiples of e) mass (amu) 0 (choose one) location on diagram x ? proton (choose one) 1.0 electron 0 (choose one) (choose one) Try Again Your answer is incorrect. • Row 2: Your answer is incorrect. • Row 3:...
Science Chemistry Q&A Library Complete the table below, using the diagram of an atom shown at right. Properties of subatomic particles approximate charge (in multiples of e) location name symbol mass on diagram (amu) 1.0 A -1 0.0005 B n° (choose one) v (choose one)♥. Complete the table below, using the diagram of an atom shown at right.
Question: Complete the table below, using the diagram of an atom shown at right. This problem has been solved! See the answer ...
Complete the table below, using the diagram of an atom shown at right. Properties of subatomic particles approximate location charge (in multiples of e) symbol mass on diagram name (amu) -1 0.0005 B 1.0, (choose one) (choose one) v :-
Question: Identifying the parts of an atom Complete the table below, using the diagram of an atom shown at right. Properties of subatomic particles charge ...









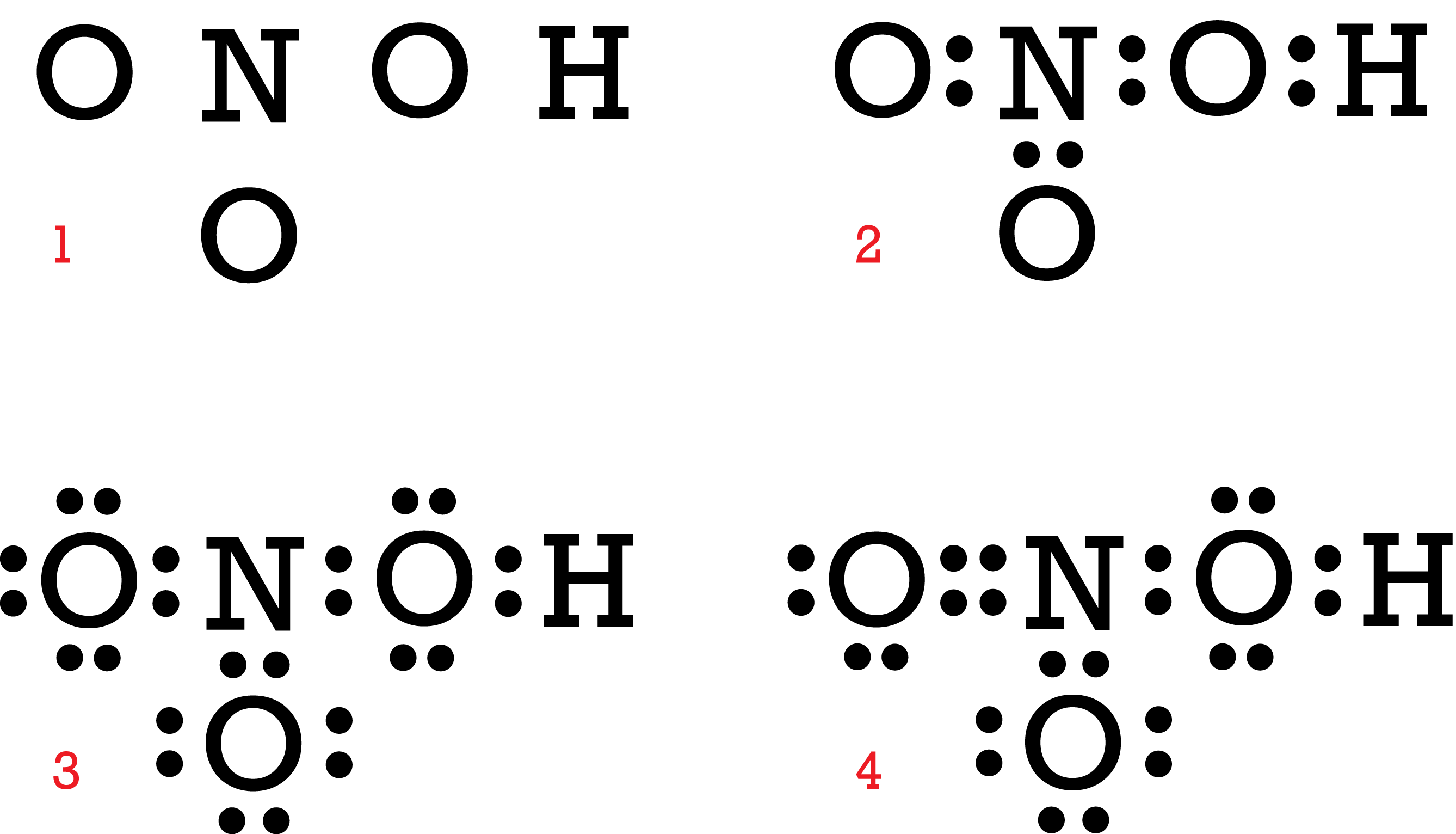








0 Response to "41 complete the table below, using the diagram of an atom shown at right."
Post a Comment