40 match the appropriate octahedral crystal-field splitting diagram
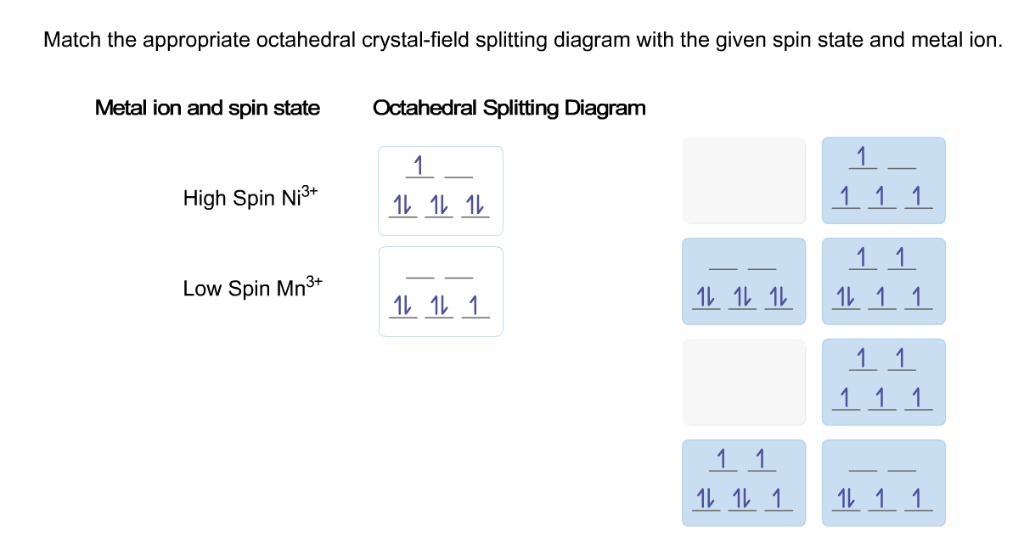
Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion Metal ion and spin state Octahedral Splitting Diagram High Spin Mn^2+ Low Spin Mn^2+ And then we have one in the upper set, so we have one at 3/5 times the octahedral crystal field splitting energy. So this ends up with minus 3/5 times the octahedral crystal field splitting energy. And notice why a is not correct--you don't have the symbol for the octahedral crystal field splitting energy.
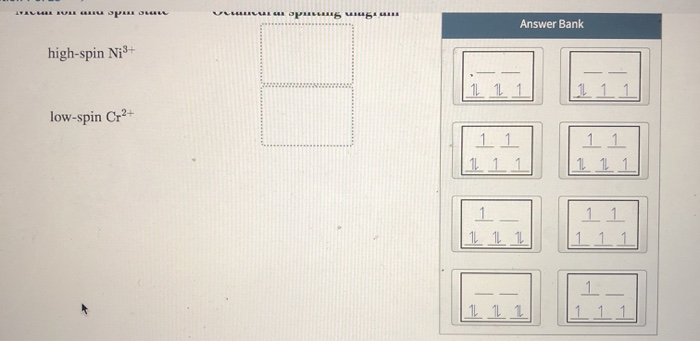
Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Mn2+ 1 11 1L 1 1 11 11 low-spin Ni3+ 11 111 1L 1.1 1L 11 11 11 11 1 |1 11 111
Match the appropriate octahedral crystal-field splitting diagram
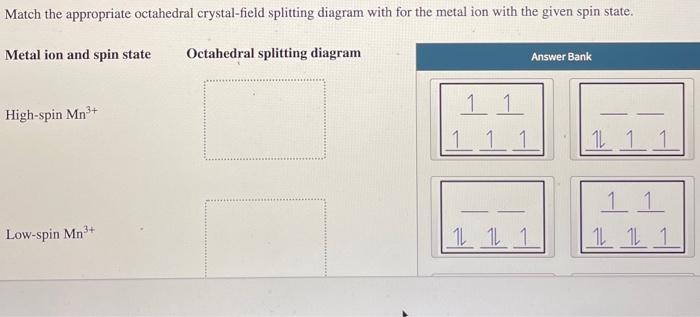
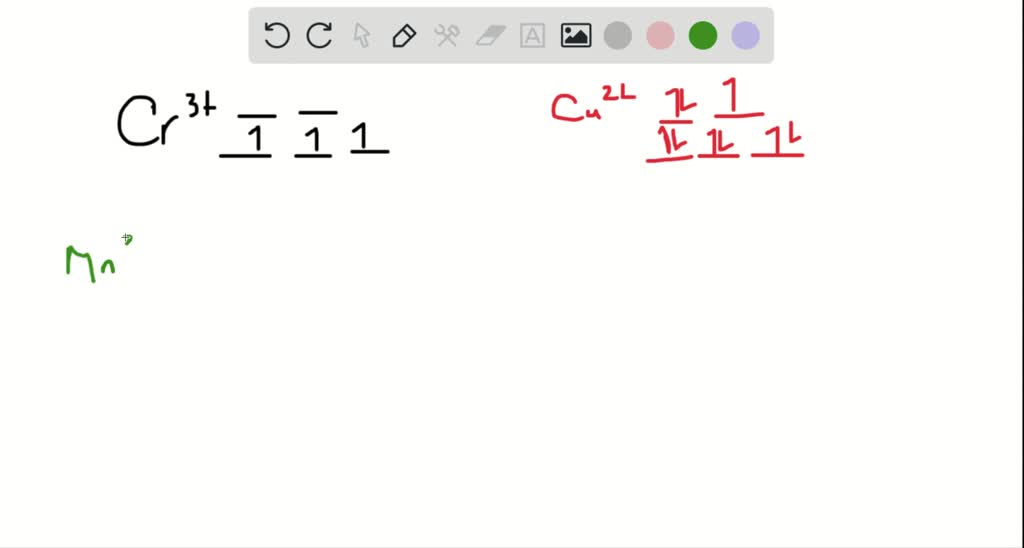
Explanation:- The valance electron configuration of Fe is 3d⁶4s². So the electron configuration for Fe3+ should be 3d⁵ …. View the full answer. Transcribed image text: Match the appropriate octahedral crystal-field splitting diagram with for the metal ion with the given spin state. Chemistry for the IB Diploma SECOND EDITION Chemistry for the IB Diploma Chemistry for the IB Diploma were given three complex signs of cobalt three that absorb light at different wavelengths. Let's match each complex sign to the appropriate wavelength, so crystal field splitting determines the difference in the energy levels of D orbital's and hence indicates the energy required for the electron transition to occur stronger than Lincoln. The more splitting there will be in the electron.
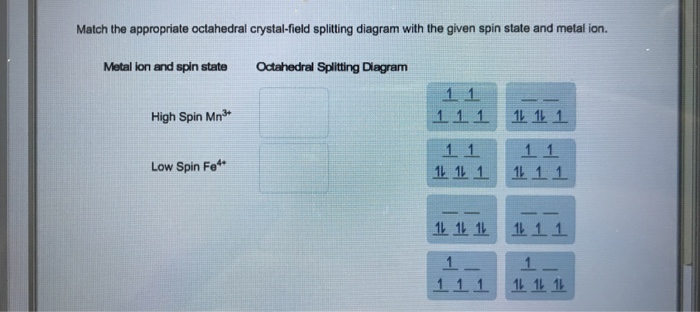
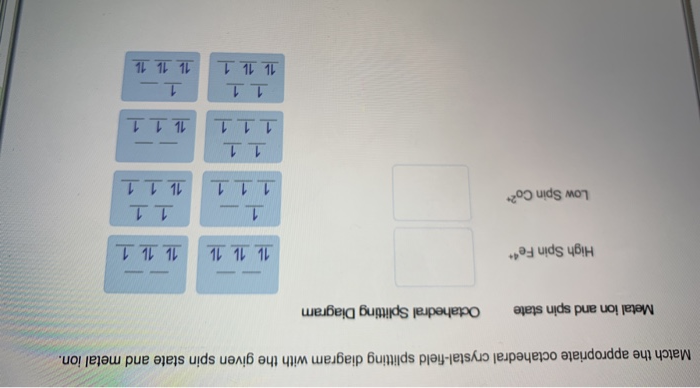
Match the appropriate octahedral crystal-field splitting diagram. CRYSTAL-FIELD SPLITTING DIAGRAMS All four of these transition metals commonly have coordination numbers of \mathbf(6), however, so let's examine their octahedral complex crystal-field splitting diagrams. HIGH SPIN VS. LOW SPIN High spin = fill all five d orbitals with one electron first, and then double up. Low spin = fill lowest-energy d ... Ans. If the element present in high spin …. View the full answer. Transcribed image text: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Fe4+ 1 1 1 1 1 1 1 1 low-spin Co2+ 1 1 1 1 1 1 11 1 1 1 1 1 1 1 1 1 1. Get the detailed answer: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. High Spin Mn^3 + Low Spi Transcribed image text: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion Metal ion and spin state Octahedral Splitting Diag High Spin Mn? 1 1 Low Spin Co2 1L 1L 1L 1L 1L 1L 1 1 1 1 1
May 03, 2021 · In addition, the splitting of the majority and minority bands (~0.2 eV at Г point) with distinct photon-energy responses has been observed by in-situ angle-resolved photoemission spectroscopy ... Lecture notes in General and Inorganic Chemistry provides an introduction to the chemistry of inorganic molecules. The emphasis is on basic principles of atomic and molecular structure, thermodynamics, chemical kinetics and catalysis, properties of Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Fe3+ 1 1 low-spin Ni4+ Dec 27, 2021 · On the other hand, in [WCl 4 (PMePh 2) 2], the octahedral symmetry is broken, which leads to further splitting of F 3/2,g while no transitions are symmetry forbidden. In the L 2-edge spectra of octahedral WCl 6 (Figure 14b), only two signals at 11561.7 eV (E 1/2,u → F 3/2,g) and 11566.0 eV (E 1/2,u → F 3/2,g ′) are present.
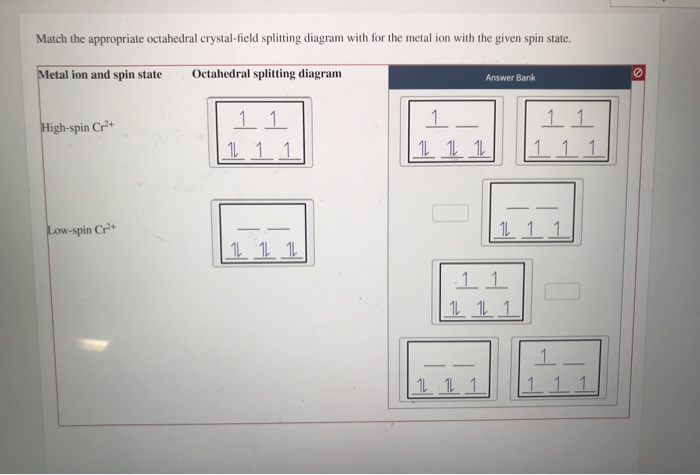
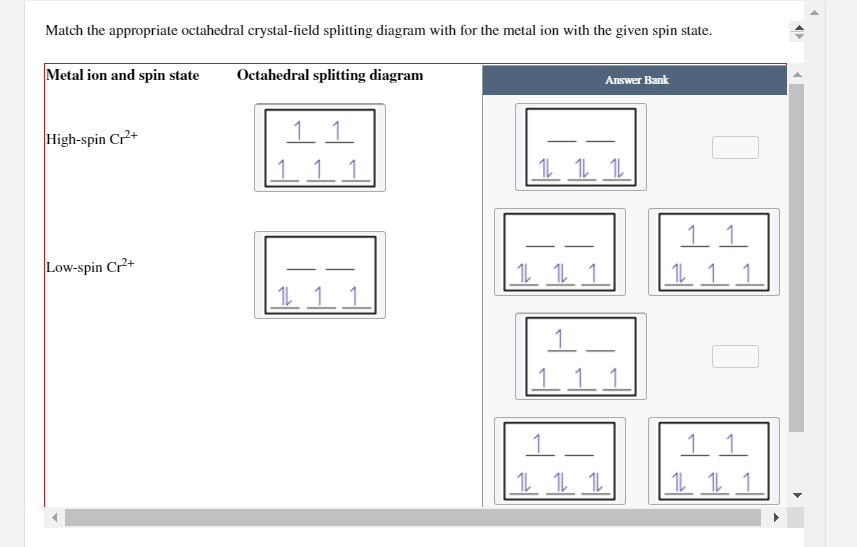
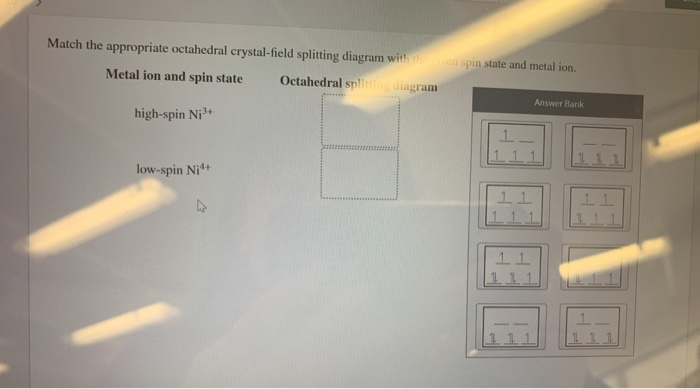
Chemistry questions and answers. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Ni3+ low-spin Fe4+. ChemistryQ&A LibraryMatch the appropriate octahedral crystal-field splitting diagram with for the metal ion with the given spin state. Metal ion and spin state Octahedral splitting diagram Answer Bank High-spin Cr+ 11 1 1 1 11 Low-spin Cr+ 11 11 1.11 |111 111 1 11 111 1L 11.1 Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Mn2+ 1 1 1 1 1 1 1 1 1. 11 11 11 low-spin Mn2+ 1L 1L 1 1 1 1 1 1 1 11 1 1 1 1 1 Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral Splitting Diagram High Spin Fe^3+ Low Spin Co^2+
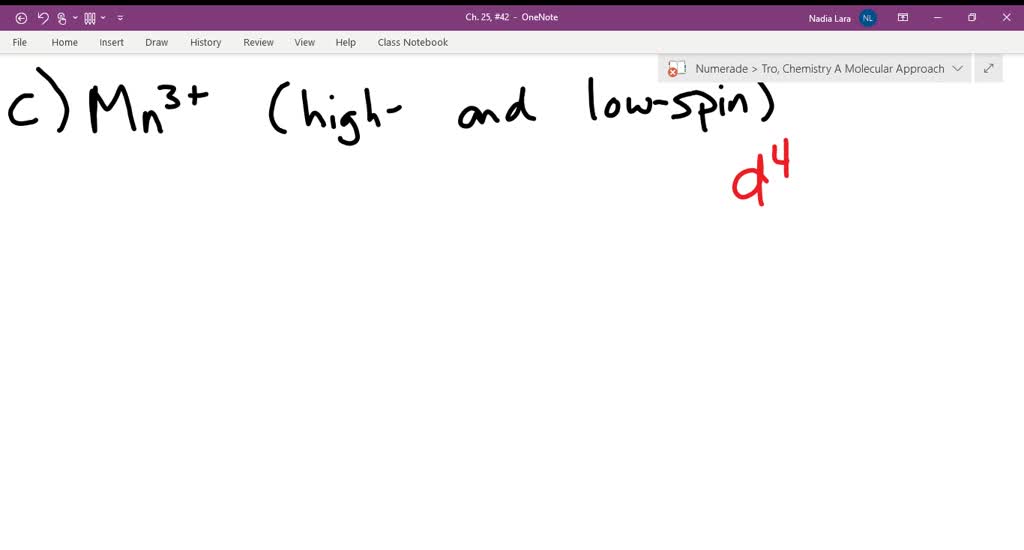
Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. High-spin Mn^3+ Low-spin Fe^3+ Asked about 2 years ago • Chemistry → Crystal Field Theory
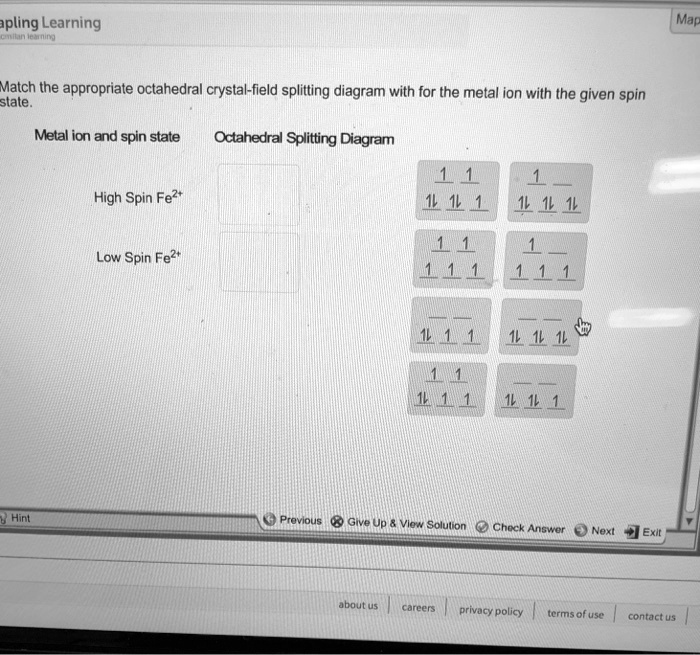
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral Splitting Diagram High Spin Ni3+ 1レ 1レ ...
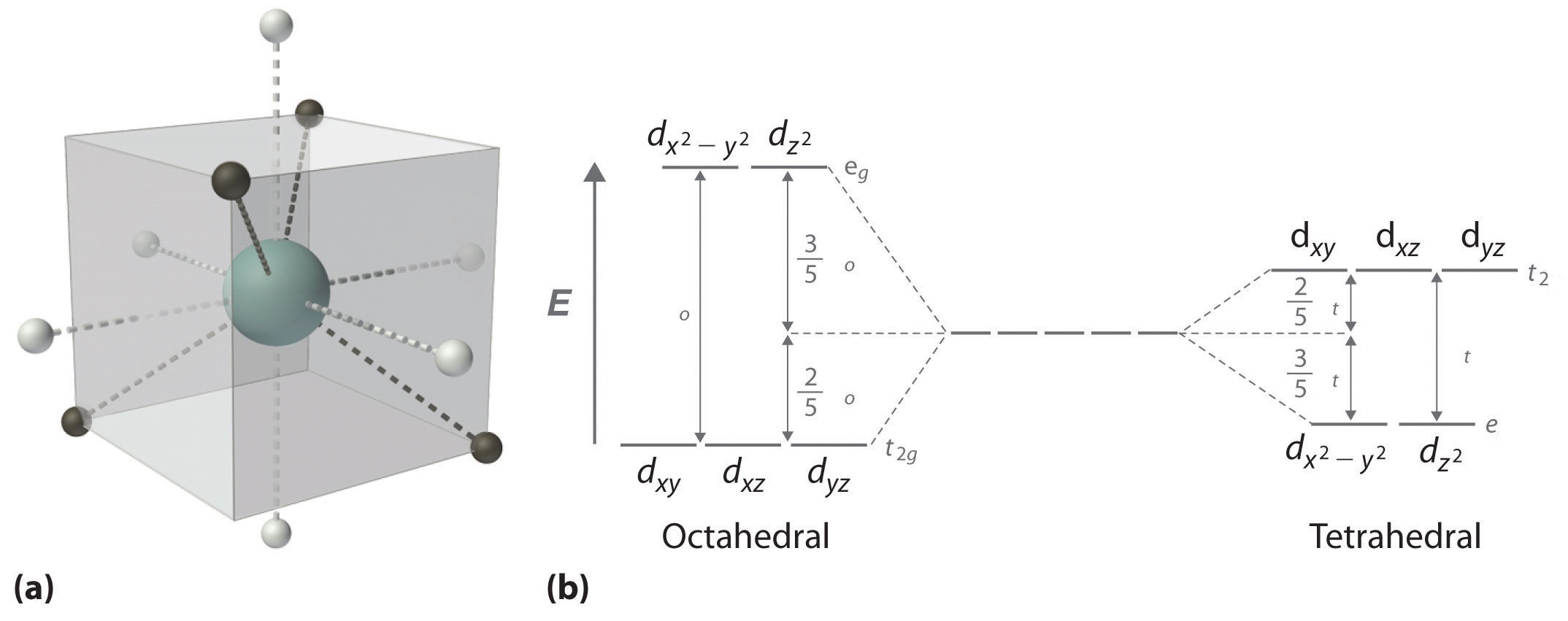
The d-orbital splitting diagram is the inverse of that for an octahedral complex. 6. 7. Page 7 of 33 Crystal Field Splitting Parameters In an octahedral or a tetrahedral crystal field, the d-orbitals are split into two sets. The energy separation between them is called the crystal field splitting parameter.
Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Weekly leaderboard. Home. Homework Help 3,800,000. Chemistry 820,000.
Knowing that halides are weak-field ligands, the maximum number of unpaired spins (i.e., high spin) is expected in this octahedral complex. That is, in an octahedral-splitting diagram, the three t2g orbitals and two eg orbitals will be singly occupied before any t2g orbitals are completely filled.
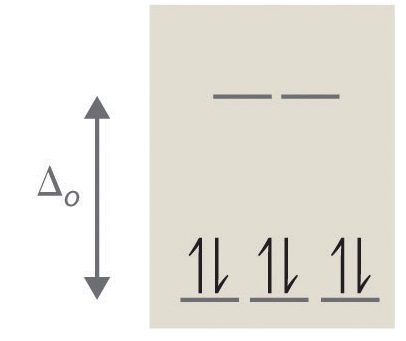
Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount of 0.6 Δo The t2gorbitals to be stabilized to the ...
Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+. A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at nm. a) Calculate the crystal-field splitting energy, Δ, in.
match the appropriate octahedral crystal field splitting diagram with for the metal ion with the given spin state metal ion and spin state octahedral splitting diagram answer bank high spin 58645
were given three complex signs of cobalt three that absorb light at different wavelengths. Let's match each complex sign to the appropriate wavelength, so crystal field splitting determines the difference in the energy levels of D orbital's and hence indicates the energy required for the electron transition to occur stronger than Lincoln. The more splitting there will be in the electron.
Chemistry for the IB Diploma SECOND EDITION Chemistry for the IB Diploma Chemistry for the IB Diploma
Explanation:- The valance electron configuration of Fe is 3d⁶4s². So the electron configuration for Fe3+ should be 3d⁵ …. View the full answer. Transcribed image text: Match the appropriate octahedral crystal-field splitting diagram with for the metal ion with the given spin state.







![Determine the CFSE in terms of Delta and P for [Co(NH3)6]3+ ...](https://study.com/cimages/multimages/16/cfse901198885569368472.jpg)
![Ni(NH3)6]Cl2 [(CH3CH2)4N]2CoCl4 Draw the crystal field ...](https://img.homeworklib.com/questions/9fdb4770-d38b-11ea-b837-8b987458f0bc.png?x-oss-process=image/resize,w_560)











0 Response to "40 match the appropriate octahedral crystal-field splitting diagram"
Post a Comment