40 liquid liquid phase diagram
Liquid-liquid phase separation (LLPS) is involved in the formation of membraneless organelles (MLOs) associated with RNA processing. The RNA-binding protein TDP-43 is present in several MLOs, undergoes LLPS, and has been linked to the pathogenesis of amyotrophic lateral sclerosis (ALS). Among the phase diagrams exhibiting eutectic behavior, the simplest one is shown in Fig. 14.3-a. The temperatures at points A and C represent the freezing Only homogeneous liquid phase exists over the curve AEC. The curve AE represents the liquidus curve on which pure solid 1 is in equilibrium with...
liquid-gas critical pressure (in the range from 0.5 to 2.0 GPa), exhibits a LLPT indicated by a discontinuity in density from a low-density liquid (LDL) to a In this work, we obtain a detailed phase diagram of the model using the values of the square-well depths and widths illustrated in Figs.

Liquid liquid phase diagram
The liquid phase will be approximated by a regular solution model. For pedagogical pur-poses, the same approximation will be made for the solid phase although it certainly does not represent an accurate description Fig. 3.1 An isomorphous phase diagram. 76 Phase diagrams. liquid phases. Journal of the American Chemical SocietyDOI: 10.1021/jacs.1c11051 Chen Yuan, Wenyan Jia, Ziyun Yu, Yanan Li, Min Zi, Li-Ming Yuan, and Yong Cui https://ift.tt/3f33Jtr We have studied the liquid-liquid phase diagram of sulfur+biphenyl. Larkin, Katz, and Scott [J. Phys. 71, 352 (1967)] reported a liquid-liquid phase separation with an upper critical solution point (UCSP) at 381 K, complete miscibility between 381 K and a lower critical solution point (LCSP) at 494...
Liquid liquid phase diagram. Liquid-liquid phase separation (LLPS) of biological polymers (protein, RNA) into liquid condensates has emerged as a critical phenomenon in the Our findings establish that α-Syn phase separates into liquid droplets, which act as an initial step towards α-Syn aggregation associated with PD pathology. A diagram of the phases as a function of pressure and temperature is called a phase diagram. Here are some example phase diagrams for carbon Let's observe some features of the CO2 phase diagram. First note that liquid/solid phase boundary and the liquid/gas phase boundary intersect. Is it cause in liquid you actually are going from solid to liquid phase and you need the change in temperature variable in the q=mct because in gas it's the last phase so you can increase the liquid so much until it fully vaporizes and that vapor just gets hotter bc there's no phase after and solid temp doesn't change until it's fully melted so it's staying at zero. Binary Solid-Liquid Phase Diagram. The substances that we encounter in the material world are hardly ever pure chemical compounds but rather mixtures of two or more such compounds. The three common phases of matter are the solid, liquid and vapor states.
The solid and liquid phases are in equilibrium all along this line; crossing the line horizontally corresponds to melting or freezing. Because no more than three phases can ever coexist, a phase diagram can never have more than three lines intersecting at a single point. A solution (liquid or solid) is phase with more than one component; a mixture is a material with more than one phase. usefulness of it. Phase diagrams provide a convenient way of representing which state of aggregation (phase or phases) is stable for a particular set of conditions. The basic phase diagram. What is a phase? At its simplest, a phase can be just another term for solid, liquid or gas. If you have some ice floating in water A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this... - 3 phases present (liquid a, liquid c, A). B At T2, monotectic reaction occurs: Liq. a + Liq. c à Liq. c + A. Alloy is liquid until it passes through liquidus at b: a-phase begins to form. More a is formed while passing through a+L region - compositional differences dictated by tie lines and boundaries.
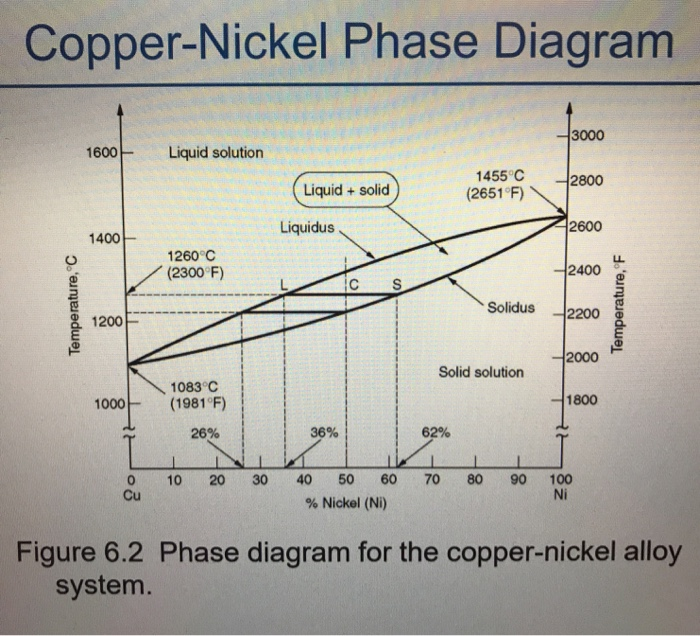
I deposited some liquidities in the Astroport liquidity pool (seeding for phase 2 of Artroport launch). But I cannot see any LP tokens deposited in my terra account, as a receipt for my deposit. I can see that my UST deposit went to the Astroport contract. Are there LP tokens on Terra? Will I receive some later, when Astroport launches? COMPONENTS, PHASES and Microconstituents. Reading phase diagrams. PHASE DIAGRAM - ex. Cu-Ni. 1500 1400 1300 1200. L (liquid) 2 liquLisdou+lisdαuαs. •AC2oxemspp?Lhona(elsinqetuss?id:) Speciαfy s(yFsCteCms coolmidpsoosiltuiotinon). what is the shape of the phase diagram of a liquid mixture containing two compounds forming AB and A2B structural solids/lattice rather than forming A and B solids/lattice when melted? I understand that congruent melting means that the solid and liquid phases have the same composition, and that in incongruent melting, the third intermediate compound decomposes upon melting. However, I am having trouble relating this to the phase diagrams that supposedly depict congruent and incongruent melting. For [congruent](https://slideplayer.com/slide/13310126/80/images/14/Compounds+formation+with+congruent+melting+point..jpg) melting: does the liquid phase then contain all three compoun...
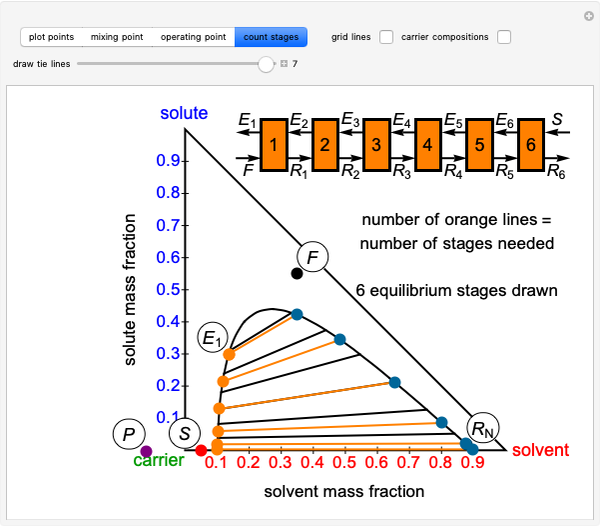
Staged Liquid-Liquid Extraction and Hunter Nash Method. = extract leaving stage . This could refer to the mass of the stream or the composition of the Locate points and on the ternary phase diagram. Connect with a straight line. Do a material balance to find the composition of one species in the...
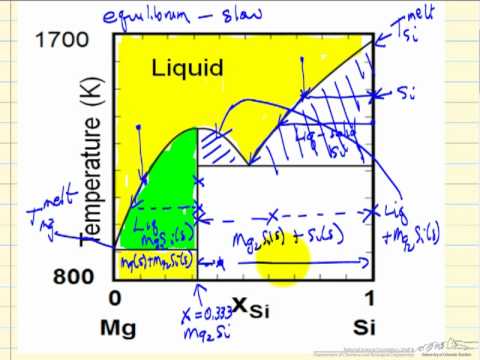
As the liquid phase changed composition with continuing removal of crystals, the successively formed crystals would become continuously more a phase diagram that has an intermediate compound that melts incongruently. a phase diagram that shows complete solid solution between two endmembers.
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically...
(phase diagram = graphical representation which indicates the phase equilibrium). • The phase diagram is used in practice to formulate systems containing more than one component where it may be advantageous to achieve a single liquid-phase product.
"white" = lighter liquid "grey" = heavier liquid Used when foams and emulsions would easily form: i.e. gentle mass transfer. Look up in your own time Once D is determined, we can obtain phase diagrams to understand how the process will operate. Also: see Perry's for many values of D. 24.
Phase diagram is a powerful tool to guide the exploitation of thermal energy materials. Heat storage technology of phase-change material (PCM) was widely used to solve major energy utilization problems on large energy consumption and low utilization efficiency. In this work, a novel solid-liquid phase...
One week ago Passive Income revealed their Passive Income 2.0 roadmap. Yesterday Passive Income made some additional announcements which are really exciting. 🔹⚖️ Passive Income will become a fully regulated DeFi company very soon. 🔹💱 With a fully regulated status Passive Income will integrate FIAT payments into their PSI-HUB mobile app which enables investors to onboard, buy and sell Passive Income with and for FIAT directly through their app. 🔹📱 The PSI-HUB mobile app will be the one-s...
Liquid-liquid phase diagrams. In the case of two liquid constituents, we may find conditions where the Gibbs energy of mixing is positive. The region below the curve represents a two-phase region. The two phases are connected by a tie-line, just like with the vapour-liquid phase diagram.
Liquids phase diagrams. Fig. 3. Vapor—liquid-phase diagram for the HCl—H2 O system (5) where (-) represents the demarcation between the 5. Vapoi—liquid phase diagram of the H2O—P20 system at 101 kPa (1 atm), where 3 is ortho, 2 pyro, and 1 meta phosphoric acid. The soHd line...
Thinking about trying this , just simpler plumbing
Consider the liquid - liquid phase diagram for nitrobenzene and normal hexane at atm and temperatures less than 300 K: Temperature (K) w2 w1 1 wNitrobenzene w3 1 phase 2 phases T1 295 K upper consulate point The upper consulate temperature is the temperature at which thermal motion...
Thus we combine the liquid composition diagram and vapor composition diagram into one. A point in the two-phase region of a phase diagram indicates not only qualitatively that the liquid and vapor are present, but represents quantitatively the relative amounts of each.
11.6 PHASE DIAGRAMS. The equilibrium between a liquid and its vapor is not the only dynamic equilibrium that can exist between states of matter. The phase diagram for any substance that can exist in all three phases of matter is shown in FIGURE 11.27.
Draw vapor-liquid equilibrium (VLE) phase diagrams for single compounds or binary systems; residue curves for ternaries. VLE-Calc.com Vapor-Liquid Equilibrium Calculator.
Liquid-Liquid Phase Diagrams. 3 215 просмотров 3,2 тыс. просмотров. Cliff Brangwynne (Princeton & HHMI) 1: Liquid Phase Separation in Living Cells.
Liquid-liquid phase separation (LLPS) is a thermodynamically-driven, reversible phenomenon consisting in de-mixing into two distinct liquid phases, with different solute concentrations. A phase diagram can be constructed by systematically screening, for instance, temperature and concentration...
A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature...
• Totally miscible liquids constitute a single phase. • In an immiscible liquid system, each layer is counted as a separate phase. Phases diagrams of some two component solid liquid equilibria are simple while those of others are quite complicated. The complex ones may be considered to be made...
Let's assume the water is pure H20 (and not seawater). How deep could this body of water be before the water pressure is great enough to phase change? What would the water look like at that depth? What type of ice would form? Would average seawater change this answer?
h Phase diagrams (constitutional diagrams) exhibit pictorial representation of relationships and product description. They describe the areas where there is liquid, a solid solution, an intermetallic compound, etc. h Phase diagrams show the equilibrium phases existing in an alloy at various...
We have studied the liquid-liquid phase diagram of sulfur+biphenyl. Larkin, Katz, and Scott [J. Phys. 71, 352 (1967)] reported a liquid-liquid phase separation with an upper critical solution point (UCSP) at 381 K, complete miscibility between 381 K and a lower critical solution point (LCSP) at 494...
Journal of the American Chemical SocietyDOI: 10.1021/jacs.1c11051 Chen Yuan, Wenyan Jia, Ziyun Yu, Yanan Li, Min Zi, Li-Ming Yuan, and Yong Cui https://ift.tt/3f33Jtr
The liquid phase will be approximated by a regular solution model. For pedagogical pur-poses, the same approximation will be made for the solid phase although it certainly does not represent an accurate description Fig. 3.1 An isomorphous phase diagram. 76 Phase diagrams. liquid phases.





















0 Response to "40 liquid liquid phase diagram"
Post a Comment