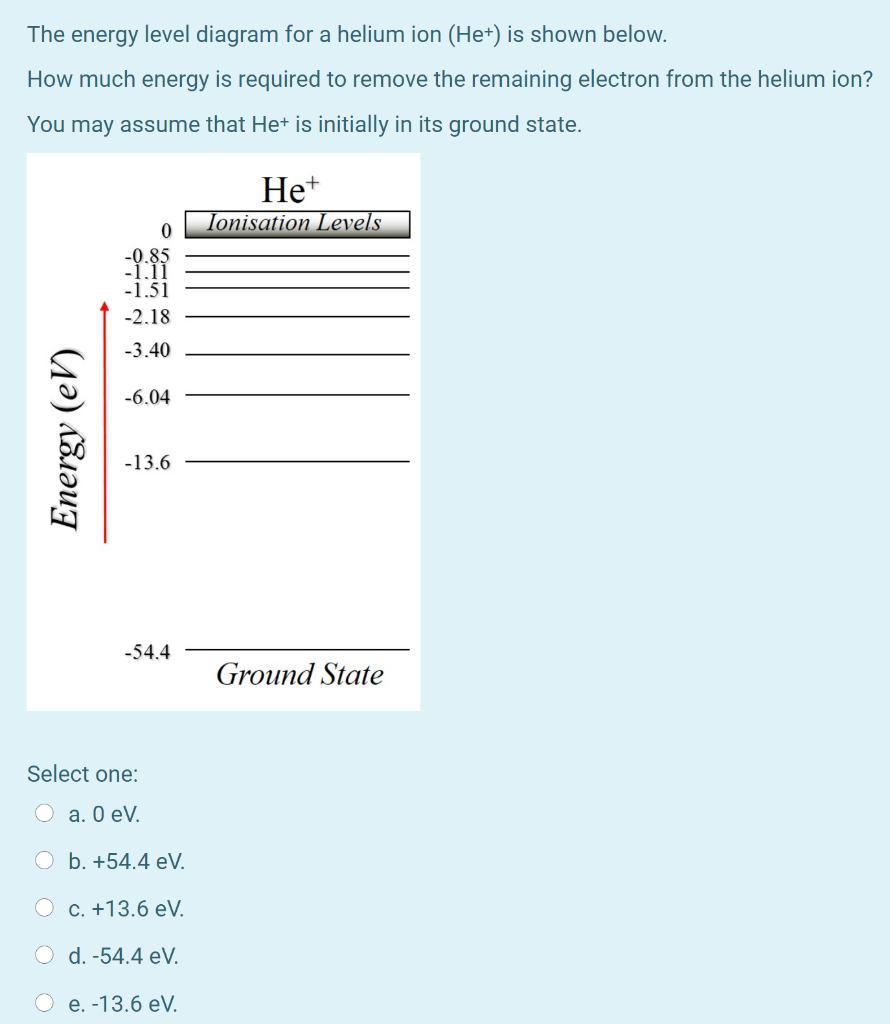
42 energy level diagram for helium
氫(拼音:qīnɡ,英語: Hydrogen ),是一種化學元素,其化學符號为H,原子序數为1,原子量為 1.007 94 u ,是元素週期表中最輕的元素。 單原子氫(H)是宇宙中最常見的化學物質,佔重子總質量的75% 。 等離子態的氫是主序星的主要成份。 氫的最常見同位素是「氕」(此名稱甚少使用,符號為 ... Components of the Atmosphere. The atmosphere is a thin layer of gases surrounding the surface of a planet. It is held in place by gravity, and typically contains a variety of gases. In the case of ...
In this paper the electrokinetic characteristics of helium low-voltage beam discharge plasma in operating conditions of a three-electrode device with a hot cathode are studied. A method and a device are proposed to ensure effective voltage stabilization in a range up to 110 V by controlling the electron velocity distribution function using the plasma channel external boundaries.
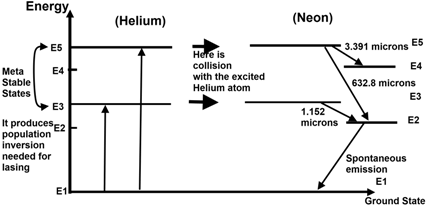
Energy level diagram for helium
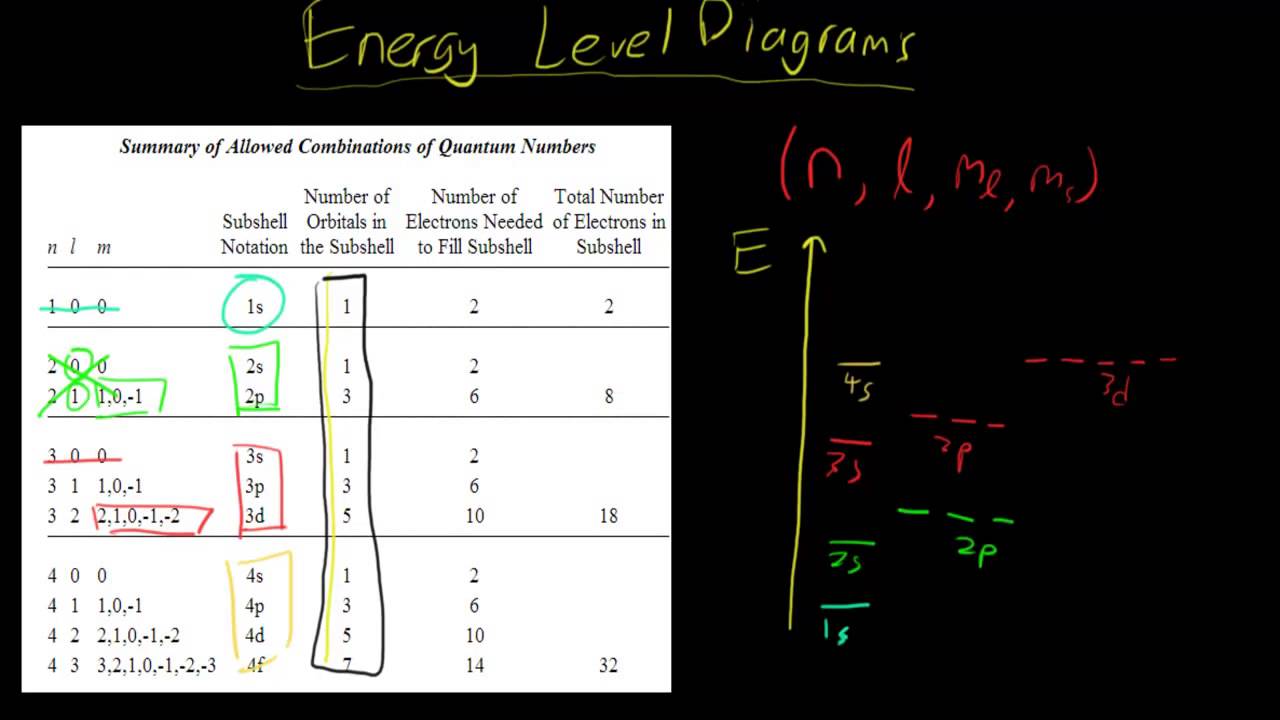
In this configuration we note that there is only one electron in the 3rd energy level. Hence the full Ground state electronic configuration for bromine in accordance with the Aufbau Principle is. What is one example for an electron configuration in the excited state for Sodium Na. Sodium atoms have 11 protons and so 11 electrons. Question: Part D Show the orbital-filling diagram for Br (bromine). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint (s) Reset Help 1 18 25 2p 3s 3p 30 48 4p Submit Provide Feedback Next > Part B Show the orbital-filling diagram for N (nitrogen). And finally you show the 4 electrons in the. Na Z 11. An electron configuration table is a type of code that describes how many electrons are in each energy level of an atom and how the electrons are arranged within each energy level. What period the element is in determines the 1st number.
Energy level diagram for helium. 1. When a SCUBA diver dives below the surface of the sea the pressure exerted on their body will increase according to the formula P=ρgh. At a depth of 100m diver blows out a bubble of air with 2cm radius. How big will this bubble be just before it breaks the surface?Hint:Solution:2. A SCUBA diver breathing air from an 18liter tank, on the surface, takes 0.5 litres of air into her lungs 20 ... Despite prolonged efforts in superfluid helium 25,26, sound-mediated dissipation of vortex energy remains elusive due to the scarcity of convincing experimental proofs 7,27. A neutral helium atom, with an atomic number of 2 (Z = 2), has two electrons. We place one electron in the orbital that is lowest in energy, the 1 s orbital. From the Pauli exclusion principle, we know that an orbital can contain two electrons with opposite spin, so we place the second electron in the same orbital as the first but pointing down ... Well neon and helium are noble gases and are in group 8 and have 8. Number of valence electrons in Helium. ... In most cases, electrons fill the lower- energy orbitals first, followed by the next higher energy orbital until it is full, and so on until all electrons have been placed. Atoms tend to be most stable with a full outer shell (one ...
The electron configuration for Ca 2 is the same as that for Argon which has 18 electrons. The closest noble gas is Ar with an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6. It requires less energy to remove an electron from a sulfide ion than from an argon atom. Hence we can say that both are isoelectronic having the same of number of ... XeO4 Lewis Structure, Geometry, Hybridization, and Polarity. XeO4 or Xenon Tetraoxide is a chemical compound made up of Xenon and Oxygen. It is prepared by treatment of barium perxenate with anhydrous sulphuric acid. It has a molar mass of 195.29 g/mol. It is exceptional for being a stable compound of a noble gas comprising of Xenon in its ... Analysis of data is basically what we used to call data collection processing and presentation plus a bit of the evaluation. As with the other criteria this is a holistic assessment based on the whole report so you can"t pin it down as easily as you could with DCP. There are many ways of analyzing data, linearizing and drawing straight lines, curve plotting and statistical analysis, all count ... Zero-point energy (ZPE) is the lowest possible energy that a quantum mechanical system may have. Unlike in classical mechanics, quantum systems constantly fluctuate in their lowest energy state as described by the Heisenberg uncertainty principle. As well as atoms and molecules, the empty space of the vacuum has these properties. According to quantum field theory, the universe can be thought ...
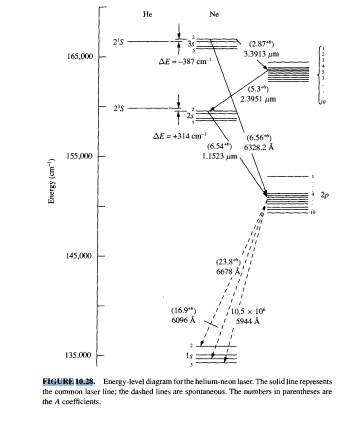
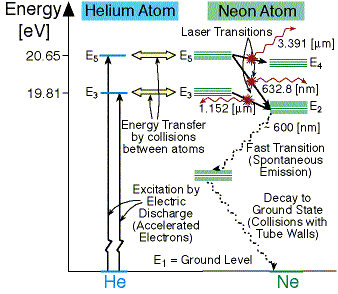
(9%) Problem 11: The figure shows the electron energy level diagram during a collision between a helium and a neon atom, which occurs in helium-neon lasers. A population of electrons is created in the. 0 answers To stop a car, first you require a certain reaction time to begin braking; then the car slows at a constant rate. Suppose that the ... Atom Sequence Diagram Examples; Atom Diagram Chart; Atom Diagram List; Sequence Diagram Online; The aufbau principle, from the German Aufbauprinzip (building-up principle), also called the aufbau rule, states that in the ground state of an atom or ion, electrons fill atomic orbitals of the lowest available energy levels before occupying higher levels. Seznamy 32+ Argon Atom Diagram. 14.08.2018 · bohr model of argon atom potassium atom, copper atom, atom model project, bohr.Download scientific diagram | energy level diagram of the argon atom. I show you where argon is on the periodic table and how to determine how m. 25.07.2019 · argon has 2 electrons in its first shell, 8 in its second, 8 in its third.check me out: An energy level can be measured by the amount of energy needed to unbind the electron from the atom, and is usually given in units of electronvolts (eV). The lowest energy state of a bound electron is called the ground state, i.e. stationary state , while an electron transition to a higher level results in an excited state. [91]
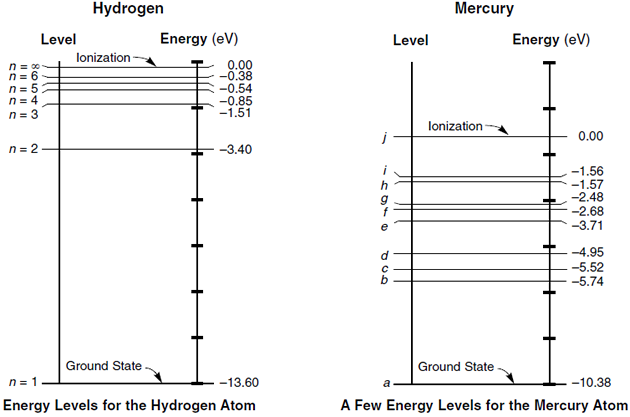
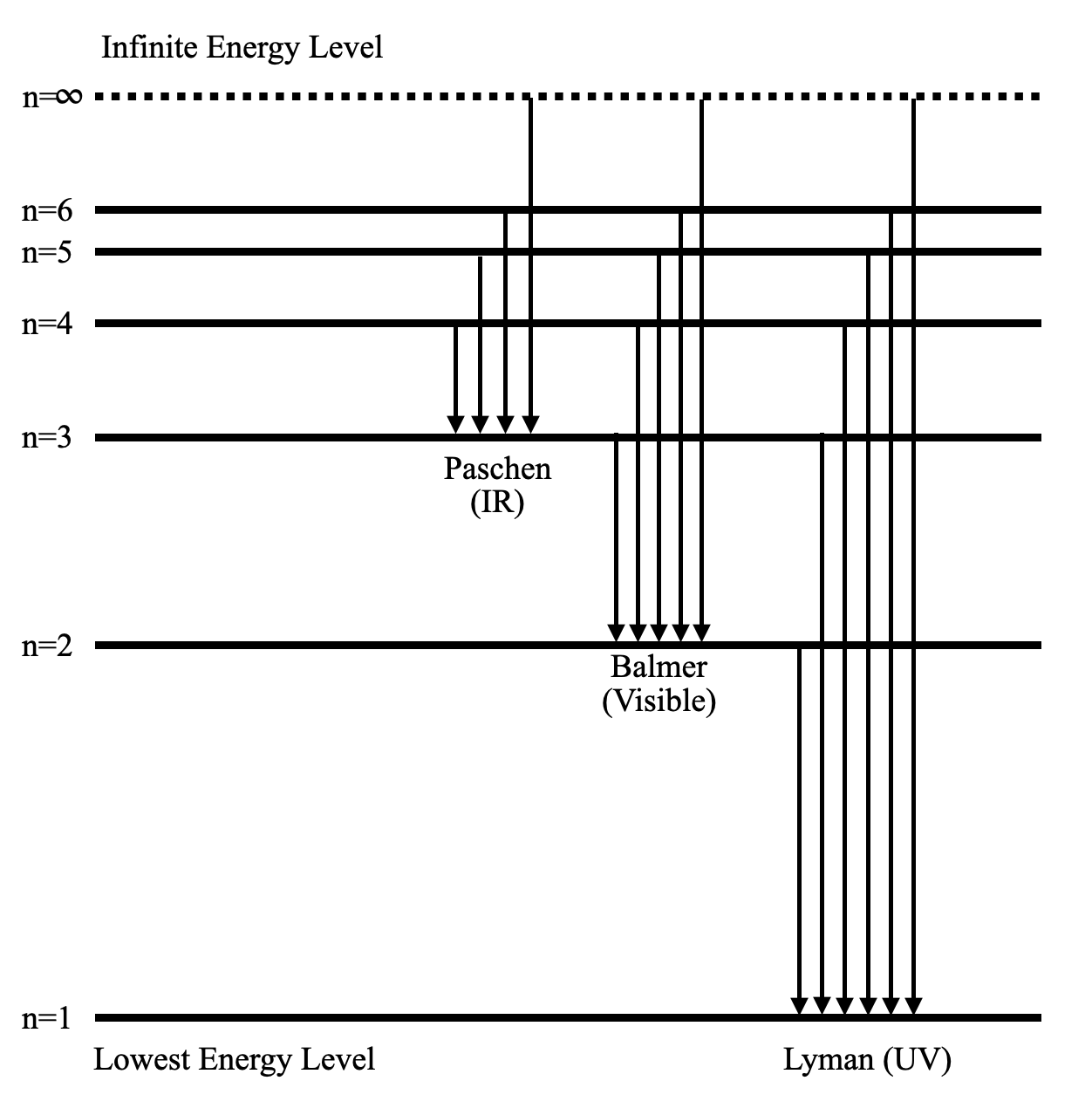
The energy of allowed orbits for hydrogen is shown to the right; we have also indicated the value of n for three of those energy levels. Technical note: the fact that the energies are negative is only due to a common convention as to where we choose the zero point of energy in a non-relativistic analysis. Recall Balmer's formula:
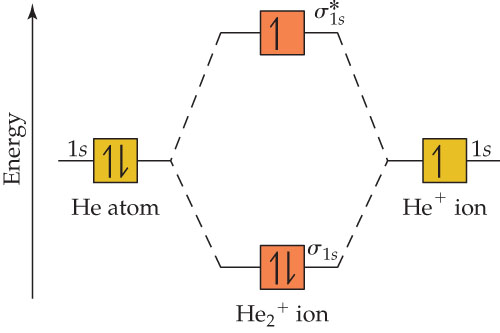
Molecular Orbital s of the Second Energy Level. The 2s orbital s on one atom combine with the 2s orbital s on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbital s formed from the 1s atomic orbital s. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbital s ...
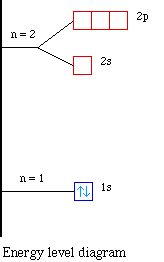
For example, 1n represents the first energy level located closest to the nucleus. Figure \(\PageIndex{5}\): The Bohr model was developed by Niels Bohrs in 1913. In this model, electrons exist within principal shells. An electron normally exists in the lowest energy shell available, which is the one closest to the nucleus.
Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine. By the end of this section, you will be able to: Describe the structure of atoms and the components of nuclei; Explain the behavior of electrons within atoms and how electrons interact with light to move among energy levels
Though, all three molecular orbital (MO) diagram s are able to explain the nature of metal-4 (ii) Complete the diagram to show the splitting of the d orbital energy levels in an octahedral complex ion. energy (iii) On the axes below, sketch the shapes of one d orbital from the lower energy level and one d orbital from the higher energy level. y ...
The bridging tren ligand pre-organized the metal ions in a trigonal manner, favorable to a µ 3-k 2:k 2:k 2 coordination mo de of the carbonate anion and this basic ligand is easy replaceable by the carbonate anion. The luminescent properties of the Cd(II) complexes may combine the sequestering abilities of such complexes with a sensing application.
5. Ionization Energy. Ionization energy is the energy required to remove an electron from a neutral atom ; I onization energy generally increases along a Period due to increase in the positive charge of the nucleus (see atomic size) Ionization energy decreases down a Group as valence electrons are in higher Principal Energy levels.
Lewis structures or Lewis dot diagrams of compounds is a simple yet effective method of indicating the chemical bonds, especially the covalent bonds between the atoms of a compound. ... except hydrogen and helium, want a total of 8 electrons, that is the octet. Hydrogen requires only two electrons. ... that is in the same energy level, interact ...
Dieke, Spectra and Energy Levels of Rare Earth Ions in Crystals (Wiley, New York, 1968). Comparison of the energy splitting of multiplets in two matrices, namely, in KDy(WO 4 ) 2 and LaCl 3 , allows us to conclude that the crystal-field strength in the first matrix is higher than in the second one, but energy shift is due to the so-called nephe ...
The electron shell or energy level farthest from the nucleus is referred to as the valence shell. The electrons residing in the valence shell are called valence electrons. In simple terms, the atom's outermost electron shell is known as valence shells, and the electrons found there are termed valence electrons.
Each orbit or shell has a fixed energy and these circular orbits are known as orbital shells. The energy levels are represented by an integer (n=1, 2, 3…) known as the quantum number. This range of quantum number starts from nucleus side with n=1 having the lowest energy level.
And finally you show the 4 electrons in the. Na Z 11. An electron configuration table is a type of code that describes how many electrons are in each energy level of an atom and how the electrons are arranged within each energy level. What period the element is in determines the 1st number.
Question: Part D Show the orbital-filling diagram for Br (bromine). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint (s) Reset Help 1 18 25 2p 3s 3p 30 48 4p Submit Provide Feedback Next > Part B Show the orbital-filling diagram for N (nitrogen).
In this configuration we note that there is only one electron in the 3rd energy level. Hence the full Ground state electronic configuration for bromine in accordance with the Aufbau Principle is. What is one example for an electron configuration in the excited state for Sodium Na. Sodium atoms have 11 protons and so 11 electrons.


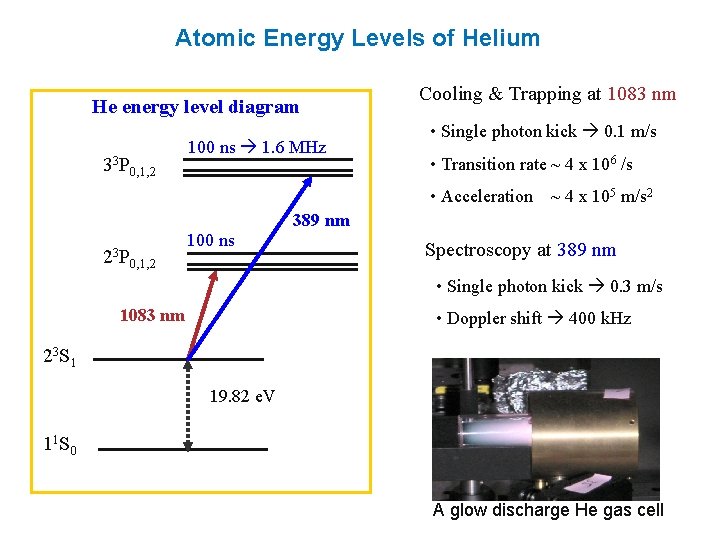




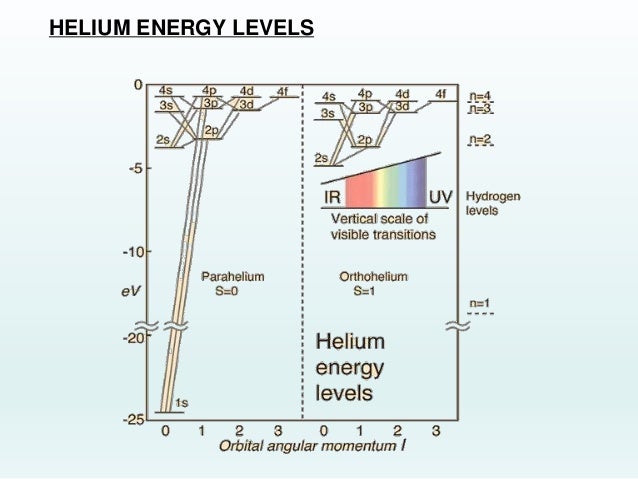
















0 Response to "42 energy level diagram for helium"
Post a Comment