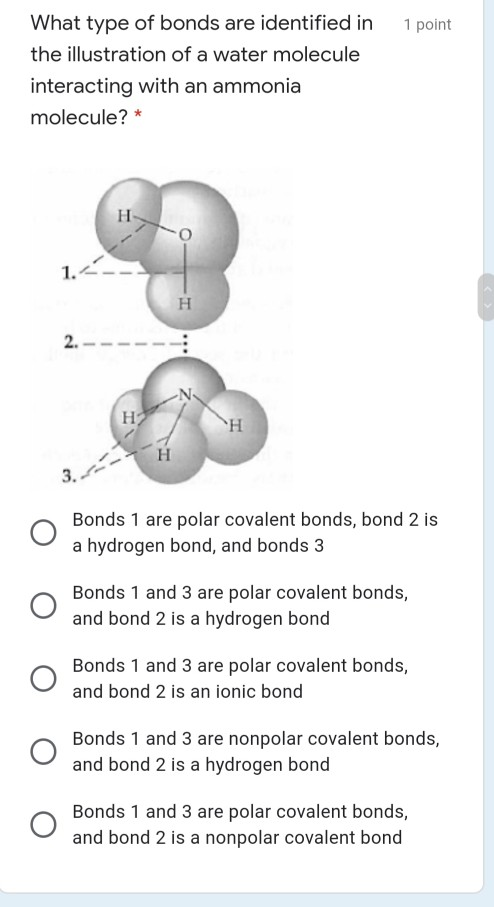
40 the ammonia molecule in the diagram has the observed bond orientation because
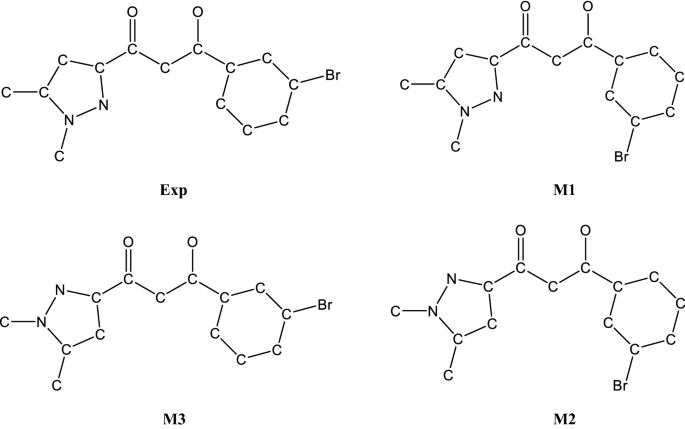
The process of chemisorption of small molecules on transition metal surfaces has been investigated from the point of view of two prototype adsorption systems, carbon monoxide on iridium {100} and {111 } and ammonia on nickel {111}. Microscopic details such as the molecular orientation on the surface, the bonding configuration and the nature of interactions between adsorbed species have been ... The ammonia molecule in the diagram has the observed bond orientation because … N has 7 protons in its nucleus. N has four pairs of electrons in the valence shell. electrons repel one another. All of the above. None of the above. All of the above. Without making or breaking bonds, the pictured molecule can change its shape because …
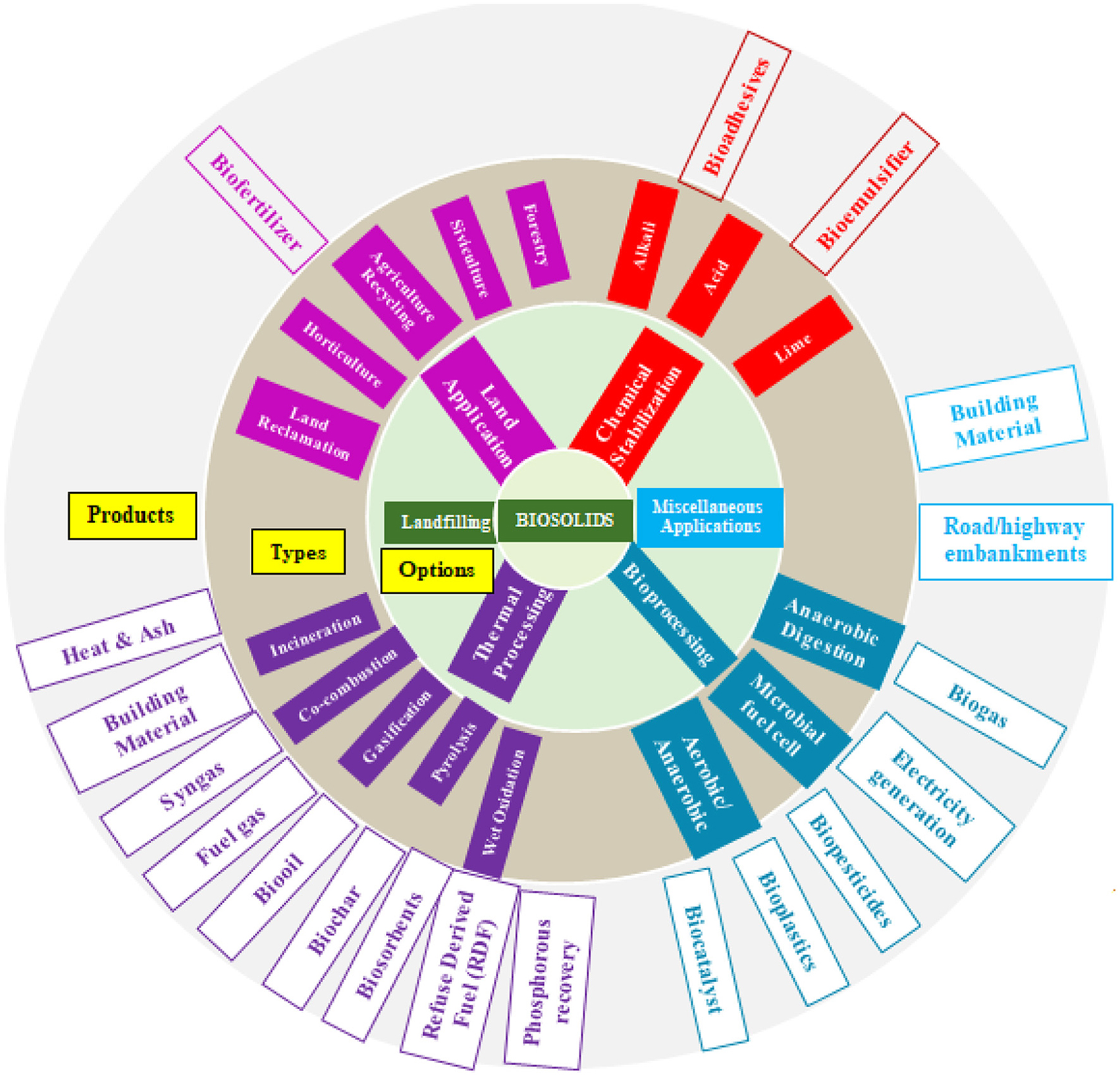
An electrically neutral molecule has the formula C3H4O2N. If the carbon atoms form the usual number of bonds, how many covalent bonds will each hydrogen atom have with other atoms in the molecule?
The ammonia molecule in the diagram has the observed bond orientation because
(a) Each CO bond has a bond dipole moment, but they point in opposite directions so that the net CO 2 molecule is nonpolar. (b) In contrast, water is polar because the OH bond moments do not cancel out. The OCS molecule has a structure similar to CO 2, but a sulfur atom has replaced one of the oxygen atoms. To determine if this molecule is ... Molecular geometry. The theory of valency which we have been developing is known as valence bond theory.One further feature of this theory is that it may be used to predict (or in some cases, rationalize) the observed geometries of molecules By the geometry of a molecule we mean the relative arrangement of the nuclei in three-dimensional space. To obtain a more accurate expression of the rate for the special case of ammonia molecule, we observed geometry optimization which leads to N-H bond length of 1.017 Å, while H-N-H bond angle is ...
The ammonia molecule in the diagram has the observed bond orientation because. Nitrogen has a total of 7 protons (its atomic number is 7) in its nucleus. Explanation: The shape and the bond orientation of molecules and ions are both explained by the valences shell electron pair repulsion theory (VSEPR). Ammonia, , is a molecule which contains three N-H bonds, as well as one lone pair on nitrogen. According to the VSEPR ... The ammonia molecule in the diagram has the observed bond orientation because a. (c) The electrostatic potential diagram of the water molecule. The polarity of the NOH bonds occurs because nitrogen has a greater electronegativity than hydrogen. (b) The dipole moment of the ammonia molecule oriented in an electric field. Ammonia, , is a molecule which contains three N-H bonds, as well as one lone pair on nitrogen. According to the VSEPR theory, molecules try to acquire a shape which would minimize the repulsion exhibited by the electron clouds present, that is, between the bonding (shared in a bond) and non-bonding (lone pair) electrons. The ammonia molecule in the diagram has the observed bond orientation because 30 more tips 1994 ford ranger fuse box diagram 30 even more tips troy bilt pony parts diagram 50 more step and image. Chap 2 Chemical Bond Chemical Polarity None of the above. The ammonia molecule in the diagram has the observed bond orientation because. Each pair of ...
The Ammonia Molecule In The Diagram Has The Observed Bond Orientation Because The electrons form 3 bonds and 1 lone pair of electrons. N has four pairs of electrons in the valence shell b. Figure 27 illustrates the hydrogen bonding as observed in crystalline ammonia. The hydrogen bonds are longer than those in ice and are non-linear. Although each ammonia molecule forms hydrogen bonds with six neighbors in the crystal, only two ammonia molecules are shown here. The ammonia molecule in the diagram has the observed bond orientation because. The ammonia molecule in the diagram has the observed bond orientation because. N has four pairs of electrons in the valence shellb. Electrons repel one another c. Rotation can occur around single bonds. All of the above e. N has 7 protons in its nucleus. So, we have tried to reproduce the observed origin band rotational profile, assuming the Structure AA-I Structure AA-II Structure AA-III transition dipole moment orientation fixed with respect to A 0 (cm 1) 0.06556 0.07148 0.07270 the anisole molecule: this assumption is justified by the B 0 (cm 1) 0.03427 0.03356 0.03086 C 0 (cm 1) 0.02679 ...
The ammonia molecule in the diagram has the observed bond orientation because ... A. N has four pairs of electrons in the valence shell. B. electrons repel one another. C. N has 7 protons in its nucleus. D. All of the above. E. None of the above. The ammonia molecule in the diagram has the observed bond orientation because. N has four pairs of electrons in the valence shell b. The electrons form 3 bonds and 1 lone pair of electrons. All of the above. The ammonia molecule in the diagram has the observed bond orientation because. Electrons repel one another c. N has 7 protons in its nucleus. The ammonia molecule in the diagram has the observed bond orientation because... a) electrons repel one another b) N has four pairs of electrons in the valence shell c) N has 7 protons in its nucleus d) all of the above e) none of the above The ammonia molecule (NH 3) has three pairs of electrons involved in bonding, but there is a lone pair of electrons on the nitrogen atom.: 392-393 It is not bonded with another atom; however, it influences the overall shape through repulsions. As in methane above, there are four regions of electron density.
The Ammonia Molecule In The Diagram Has The Observed Bond Orientation Because ... 1jzgte Mk3 Supra Tachometer Wiring Diagram; Trrs Plug Wiring; Kenwood Kdc 1011s Wiring Diagram; Sensi St55 Wiring Diagram; 1999 Oldsmobile Intrigue Radio Wiring Diagram; Quadrajet Vacuum Ports Diagram; Baldor Cl3608tm Wiring Diagram; Tach Wiring Diagram For A 81 ...
View full document. The ammonia molecule in the diagram has the observed bond orientation because…. All of the above (N has 7 protons in its nucleus, electrons repel one another, N has four pairs of electrons in the valence shell) The discovery of which of the following led to a drastic change in deep sea mining?
The ammonia molecule in the diagram has the observed bond orientation because ... a. N has four pairs of electrons in the valence shell b. electrons repel one another c. N has 7 protons in its nucleus d. All of the above e. None of the above: All of the above Since N has 7 protons, it must fill the second shell, giving it 4 pairs of electrons.
A double bond involves two orbitals, each with a shared pair of electrons. The ammonia molecule in the diagram has the observed bond orientation because ... All of the above. Correct Correct! Since N has 7 protons, it must fill the second shell, giving it 4 pairs of electrons. The electrons form 3 bonds and 1
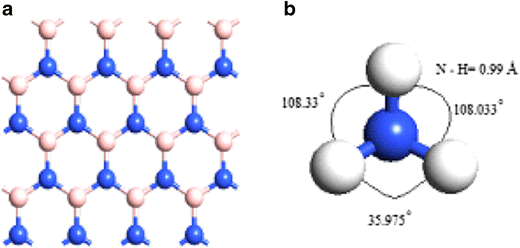
In the molecule of ammonia, the nitrogen forms three bonds with hydrogen. Now question arises whether in the formation of a molecule of ammonia, there is an overlapping of three 2p orbitals of nitrogen with three 1s orbitals of three hydrogen atoms. If this had been the case, the expected H—N—H bond angles in ammonia would have been 90°.
The ammonia molecule in the diagram has the observed bond orientation because ... N has four pairs of electrons in the valence shell. N has 7 protons in its nucleus.
To obtain a more accurate expression of the rate for the special case of ammonia molecule, we observed geometry optimization which leads to N-H bond length of 1.017 Å, while H-N-H bond angle is ...
Molecular geometry. The theory of valency which we have been developing is known as valence bond theory.One further feature of this theory is that it may be used to predict (or in some cases, rationalize) the observed geometries of molecules By the geometry of a molecule we mean the relative arrangement of the nuclei in three-dimensional space.
(a) Each CO bond has a bond dipole moment, but they point in opposite directions so that the net CO 2 molecule is nonpolar. (b) In contrast, water is polar because the OH bond moments do not cancel out. The OCS molecule has a structure similar to CO 2, but a sulfur atom has replaced one of the oxygen atoms. To determine if this molecule is ...



















0 Response to "40 the ammonia molecule in the diagram has the observed bond orientation because"
Post a Comment