45 lever rule ternary phase diagram
Lever rule applied to a binary system. Point 1; Point 2; Point 3; Point 4; Point 5; If an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever rule to the phase diagram. The lever rule can be explained by considering a simple balance. The composition of the alloy is represented by the ... Properties of ternary diagrams. Phase behavior of mixtures containing three components is represented conveniently on a triangular diagram such as those shown in Fig. 1.Such diagrams are based on the property of equilateral triangles that the sum of the perpendicular distances from any point to each side of the diagram is a constant equal to the length of any of the sides.
At first glance, there seems to be a lot going on in a two-phase diagram. There are temperatures, percentages, different elements, and symbols you're not used to seeing. But once you understand what you're looking for, everything makes sense. Here is an example of a phase diagram for an SiO₂ and Al₂O₃ mixture. On the x axis, we have the mole % of Al₂O₃ in the mixture.

Lever rule ternary phase diagram
A point in the two-phase region of a phase diagram indicates not only qualitatively that the liquid and vapor are present, but represents quantitatively the relative amounts of each. To find the relative amounts of two phases a & b in equilibrium, we measure distances on the tie line, l a and l b between the two phases and use the lever rule: A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium Lever rule. In chemistry, the lever rule is a formula used to determine the mole fraction ( xi) or the mass fraction ( wi) of each phase of a binary equilibrium phase diagram. It can be used to determine the fraction of liquid and solid phases for a given binary composition and temperature that is between the liquidus and solidus line.
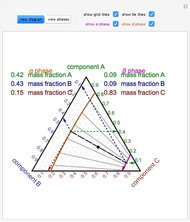
Lever rule ternary phase diagram. The point representing the composition of the final mixture would again be determined by applying the inverse-lever-arm rule along the line. Figure 5-7: Ternary Phase Diagram for a System Consisting of Components A, B, and C Which are Miscible in All Proportions. Phase relationship may also be represented on a triangular phase diagram Figure 5 ... Ternary phase diagrams are important for liquid-liquid extraction and are used in the Hunter-Nash method to determine the number of stages required for separations. This module is intended for material and energy balances, thermodynamics, and separations courses. Be able to apply the lever rule to determine the amounts of two phases in equilibrium. PHASE COMPOSITION AND LEVER RULE Lever rule: the fractional amounts of two phases are inversely proportional to their distances along the tie line (isotherm) from the bulk composition axis X f Y f L f L 1 1 2 2 L 1 = Xf L 2 = fY 1 1 2 2 1 1 1 f f L f f L 2 1 12 fY XY L f LL 1 Phase 2 "balance the teeter-totter" 459 Now that we know the compositions of the two phases, we need to find how much of each phase exists at the given temperature. The ratio of the two phases present can be found by using the lever rule.. At first sight the lever rule can appear confusing. It is really invoking the conservation of mass, and can be proved mathematically, as shown below the diagram.
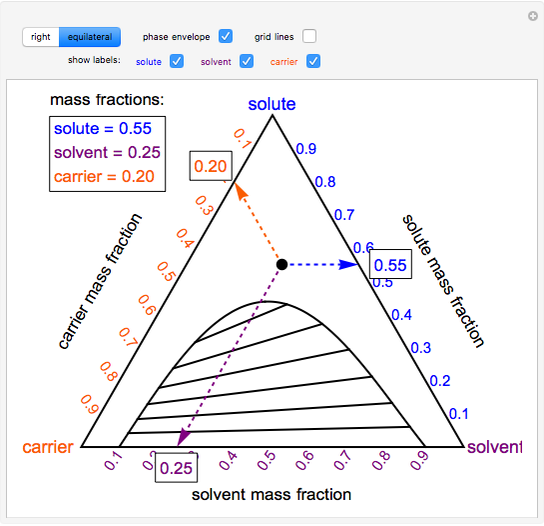
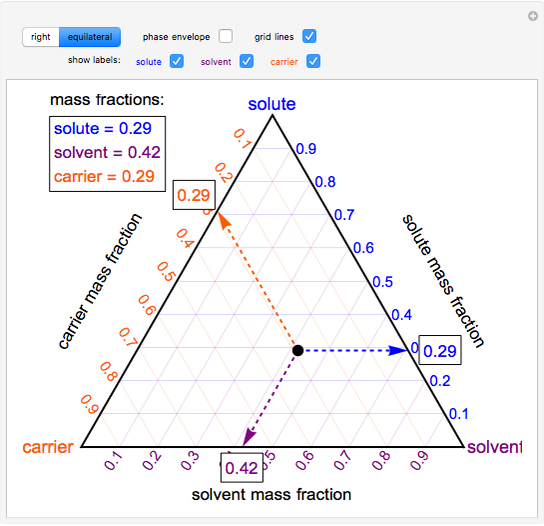
A ternary phase diagram shows possible phases and their equilibrium according to the composition of a mixture of three components at constant temperature and pressure. Figure 4.23 shows a schematic of a ternary phase diagram. Single-phase regions are areas that originate from the vertex of the triangle and that are not enclosed by black curves. Uses a simulation to show how ternary phase diagrams are interpreted. This is for a single phase, three component system. The simulation is available at: ht... Binary phase diagrams and Gibbs free energy curves • Binary solutions with unlimited solubility • Relative proportion of phases (tie lines and the lever principle) • Development of microstructure in isomorphous alloys • Binary eutectic systems (limited solid solubility) • Solid state reactions (eutectoid, peritectoid reactions) • Binary systems with intermediate phases/compounds Ternary phase diagram books by D.R.F. West - there are several . Ternary grid . ... ≡ Lever Rule . Not so useful, although . mathematically correct. Not all the compositions . Might lie in this section! Why vertical sections . are limited: Binary eutectic in a ternary system:
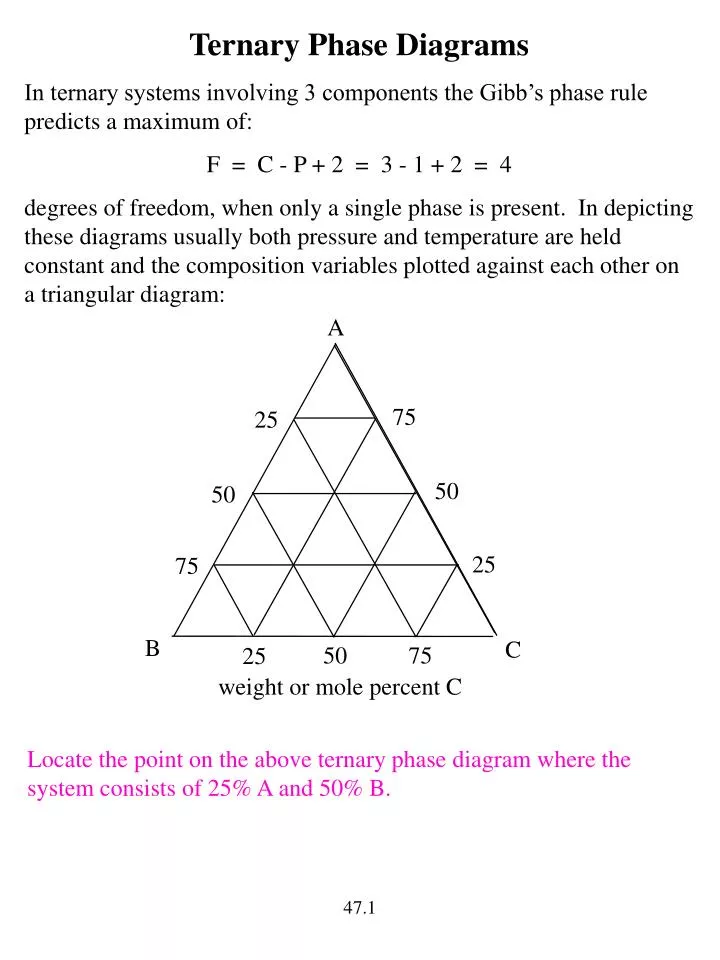
Ternary or triangular phase diagrams can be used to plot the phase behavior of systems consisting of three components by outlining the composition regions on the plot where different phases exist. Ternary phase diagrams lesley cornish. In figure 57c point m is a. By the so called inverse lever arm rule as shown in the figure 57c. I have read that to know the composition of different phases in a ternary phase diagram of metals A,B,C (at a particular temp. T), we can apply lever rule along the tie line. 50 Fresh s How to Read Ternary Phase Diagram. ternary plot a ternary plot ternary graph the first method is an estimation based upon the phase diagram grid the concentration of each species is. Applying the Phase Rule to Ternary Diagrams. ternary diagram basics ternary diagram basics engineer clearly loading mod 01 lec 25 ternary phase diagram ... Lecture Notes - The Lever Rule • The definition of the scale of a composition axis is generally chosen so that the values (e.g. ... triangular or ternary composition diagram. The attached ternary diagram is contoured (sequentially) in terms of the three plotting coordinates [B/(A+B+C)], [C/(A+B+C)], and
The lever rule is used to calculate the relative amounts of the two phases, which are represented on the bar graph on the right. The compositions of the two phases are indicated by dotted lines on the phase diagram and numerically at the top of the bar graph. Outside the curve, and mix spontaneously to form one layer.
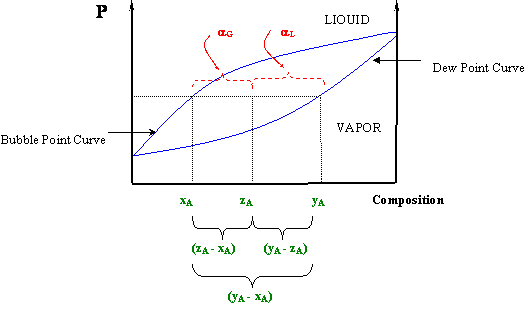
Sometimes it is also known as the "reverse arm rule," because for the calculation of α L (liquid) you use the "arm" within the (y A -x A) segment closest to the vapor, and for the vapor calculation (α G) you use the "arm" closest to the liquid. Figure 5.5: The Lever Rule In a P-x Diagram. At this point you will see clearly why we ...
5.3: The Lever Rule. P-x and T-x diagrams are quite useful, in that information about the compositions and relative amounts of the two phases can be easily extracted. In fact, besides giving a qualitative picture of the phase behavior of fluid mixtures, phase diagrams can also give quantitative information pertaining to the amounts of each ...

Fa20 Chen3100 Introduction To Gibbs Diagrams And Single Stage Lle Pdf Ternary Phase Diagrams It Is Convenient To Construct Ternary Phase Diagrams On Course Hero
3- The percentage of crystals formed at any point can be determined using the lever rule. For example, on the ternary diagram sphene - anorthite - wollastonite (Fig. 5), at a temperature represented by point "B", the % of crystals formed from a melt of composition "A" will be: % crystals = 100[AB/BC] (Fig. 5).
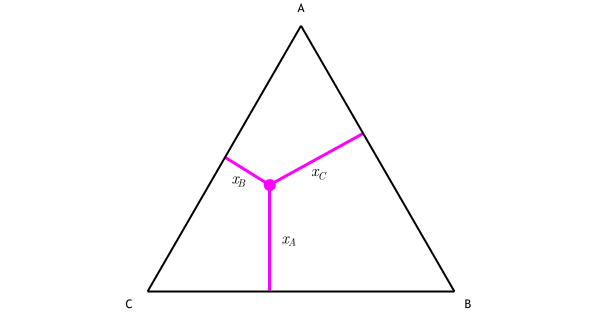
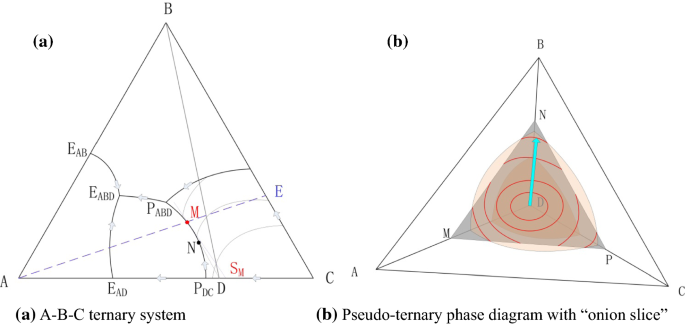
It is convenient to use Fig. 3.5 B to illustrate the application of the lever rule in ternary systems. As before, it may be of interest to know the amount of any phase present at a particular temperature, x, for a fixed bulk composition, c. Construction lines xA and xC can be drawn to construct the triangle xAC.
Applies the lever rule to a solid-liquid mixture to determine the fraction of each phase in equilibrium and explains the basis for the lever rule. Made by fa...
The lever rule. If an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever rule to the phase diagram. The lever rule can be explained by considering a simple balance. The composition of the alloy is represented by the fulcrum, and the compositions of the two phases by the ends of a bar.
Lever Rule Applied to Phase Diagram for Partially Miscible Liquids Lisa M. Goss; Ternary Phase Diagram with Alternate Phase Envelope Megan E. Maguire and Rachael L. Baumann; Tie Lines from a Conjugate Curve in Ternary LLE Diagram Housam Binous, Farrukh Shehzad, Abdelmalek Hasseine, and Ahmed Bellagi; Apply the Hunter-Nash Method to Liquid ...
The Gibbs Phase Rule for 3- Components Systems Information regarding phase equilibria can be predicted by a simple rule: F=C+2-P C is the number of components P is the number of phases present F is the degrees of freedom or variance Examples: For pure gas: C = 1 (only one component) and P = 1 (only one phase; the gas phase) F=1+2-1=2 This ...
1. Locate composition and temperature in diagram 2. In two phase region draw the tie line or isotherm 3. Fraction of a phase is determined by taking the length of the tie line to the phase boundary for the other phase, and dividing by the total length of tie line The lever rule is a mechanical analogy to the mass balance calculation.
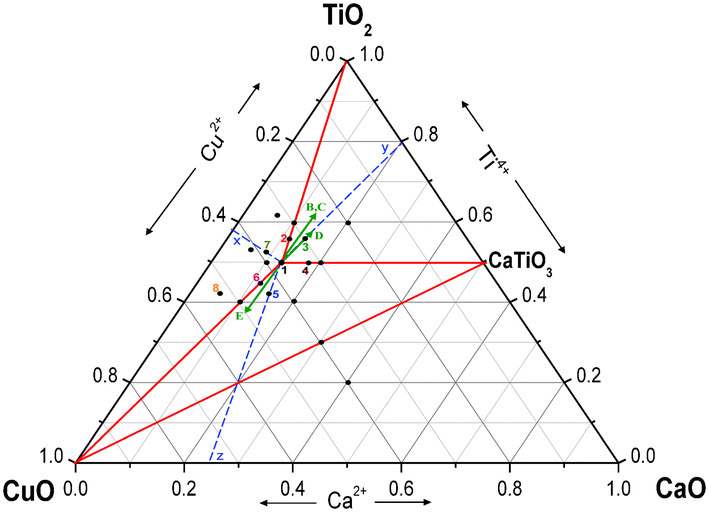
Non Stoichiometry In Cacu 3 Ti 4 O 12 Ccto Ceramics Rsc Advances Rsc Publishing Doi 10 1039 C3ra41319e
The lever rule in three phase region is graphically illustrated by the weighted phase fractions distributed about the average composition. Figure 31-5: Example of a simple ternary phase diagram at constant and .
Chapter 8 10 8.5 The Lever Rule The weight percentages of the phases in any 2 phase region can be calculated by using the lever rule Let x be the alloy composition of interest, its mass fraction of B (in A) is Cο Let T be the temperature of interest ⇒at T alloy x consists of a mixture of liquid (with C L-mass fraction of B in liquid) and solid (C S - mass fraction of B in solid phase)
Lever Rule (Interactive) Lever Rule Derivation. ... Phase Equilibrium: Txy Diagram. mirror. Plotting a Ternary Phase Diagram. mirror. Pxy Diagram for VLE (Simulation ...
Chapter 10: Ternary Phase Diagrams / 195. Fig. 10.5 . Isopleth through hypothetical ternary phase diagram at a constant . 40% . C. adapted from ref 10.1. SPB Line. The single-phase boundary line is found on any section that . contains a single-phase region. The line is what its name implies. It is the boundary line around that single-phase region.
Lever rule. In chemistry, the lever rule is a formula used to determine the mole fraction ( xi) or the mass fraction ( wi) of each phase of a binary equilibrium phase diagram. It can be used to determine the fraction of liquid and solid phases for a given binary composition and temperature that is between the liquidus and solidus line.
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium
A point in the two-phase region of a phase diagram indicates not only qualitatively that the liquid and vapor are present, but represents quantitatively the relative amounts of each. To find the relative amounts of two phases a & b in equilibrium, we measure distances on the tie line, l a and l b between the two phases and use the lever rule:

Cholesterol Induced Fluid Membrane Domains A Compendium Of Lipid Raft Ternary Phase Diagrams Sciencedirect






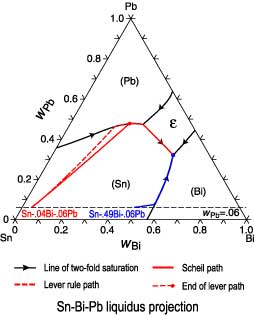
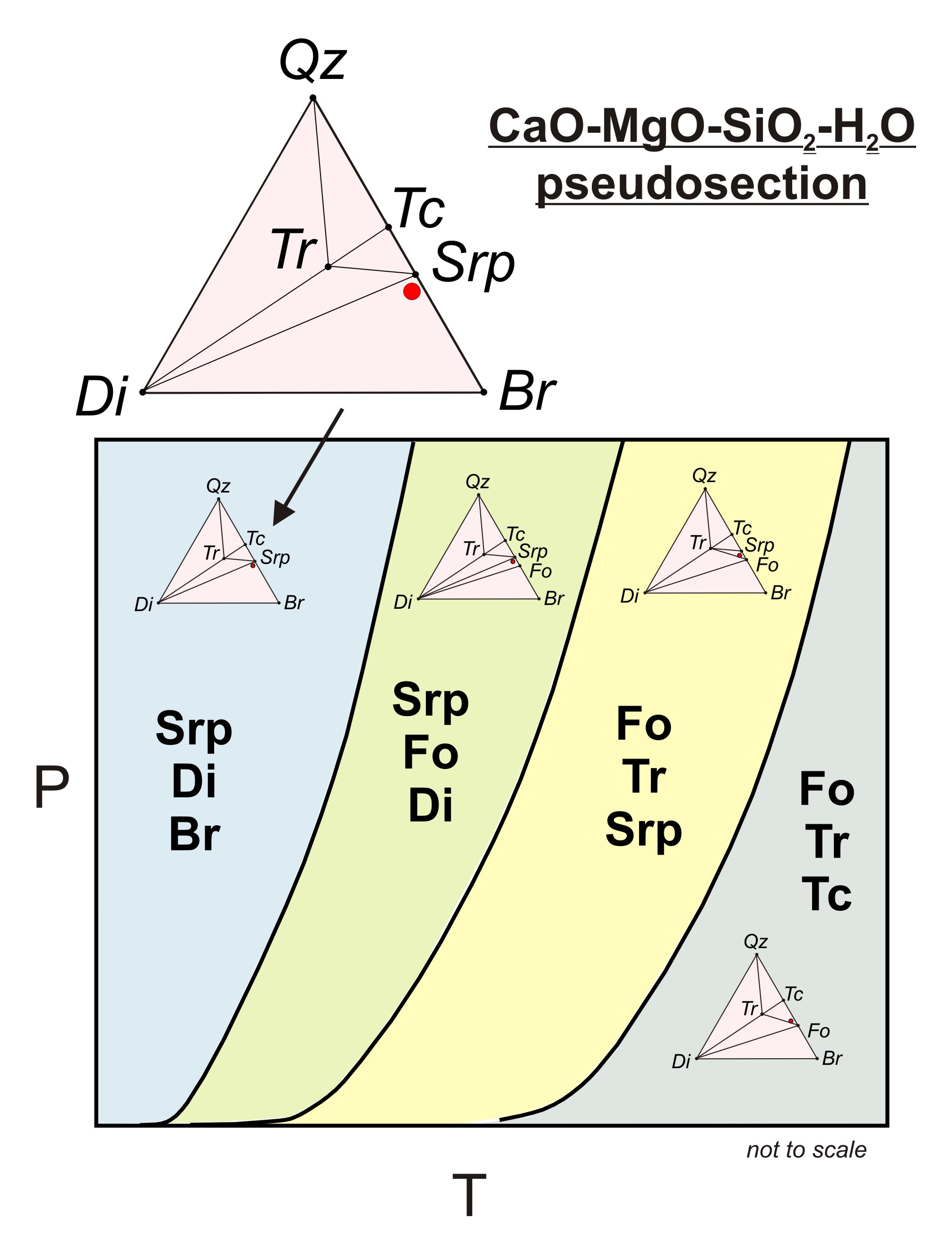









0 Response to "45 lever rule ternary phase diagram"
Post a Comment