43 dot diagram for nitrogen
NF3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. Nitrogen trifluoride or NF3 is a nitrogen halide compound that is slightly water-soluble. Its noticeable characteristics include being colorless and carrying a musty or moldy odor. NF3 has a molar mass of around 71.002 g/mol and a density of 3.003 kg/m3. It's easiest to think in terms of dots to make the N2 Lewis structure. Nitrogen needs to bond three times, shown as the lone dots on the left, right and bottom ...
The planet's most mind-blowing destinations. Fights erupted at a high school in Louisiana. So these dads took matters in their own hands. Geraldo Rivera defies colleague Tucker Carlson's push to ...
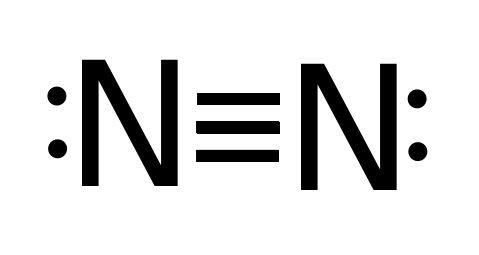
Dot diagram for nitrogen
When isolated from graphite, graphene's chemical structure looks like a honeycomb of pure carbon atoms. It's one of the thinnest materials on Earth, yet is 200 times stronger than steel. Nitrogen is in group 5 so it forms three covalent bonds. There are three shared spaces between the circles, so add a dot and cross to each one. This incomplete dot and cross diagram shows only the bonding pairs of electrons. Finally, add in the non-bonding outer electrons. Nitrogen atoms have five outer electrons. 13+ Dot And Cross Diagram. An ionic compound is formed by massive numbers of positive and negative ions. The dot and cross products. A structural formula in which electrons are represented by dots; A dot diagram (also called an electron dot diagram, and a lewis structure) is a way to show the valence electrons that surround an element.
Dot diagram for nitrogen. Using electron-dot diagrams which show only the outermost shell electrons show how a molecule of nitrogen N2 is formed from two nitrogen atoms. asked Aug 14, 2019 in Class X Science by navnit40 Expert (40.4k points) metals and non-metals. 0 votes. 1 answer. 2:30How to Draw the Lewis Structure of N2 - with explanation! ... Made Easy: Examples and Tricks for Drawing ...Sep 11, 2014 · Uploaded by chemistNATE 14+ Electron Dot Structure Of Nh3. Alternatively a dot method can be used to draw the nh3 lewis structure. We're going to do the lewis structure for nh3: NH3 Lewis and 3-D Structure- Dr. Sundin - UW-Platteville from people.uwplatt.edu Nitrogen has 5 valence electrons, but notice that nh4+ is a… Describe the electron period diagram device of representing structure.Draw electron period diagrams because that elements. You are watching: How many dots are there in the lewis symbol for a nitrogen atom, n? How execute we show electrons in atoms? Diagrams save a lot of valuable information in a compact format. What go the diagram over tell us?
A natural CO2-sink thanks to symbiotic bacteria. by Max Planck Society. A researcher from the Max Planck Institute for Marine Microbiology taking samples in seagrass meadows in the Mediterranean ... fahrbot-bot writes: A research team at the University of Wisconsin-Madison has identified a new way to convert ammonia to nitrogen gas through a process that could be a step toward ammonia replacing carbon-based fuels. The discovery of this technique, which uses a metal catalyst and releases—rather than requires—energy, was reported Nov. 8 in Nature Chemistry and has received a provisional ... Draw the dot diagram and . Save Image. AS.5 Bonding Chemistry 1 with Rob at Ashton Sixth Form . Save Image. what is the electron dot structure of CO2 and H2O2 . ... NEW NITROGEN DIOXIDE BONDING STRUCTURE nitrogen. Save Image. Draw Lewis dot diagram for the following. Carbon dioxide . Crystal structure of β-Gallium oxide. Credit: Orci/Wikimedia Commons CC BY-SA 3.0 As a crucial part of spectrum analysis, solar-blind ultraviolet photodetectors (SBPDs) are applied to many fields.
Mesoporous structure enhances catalytic performance of single-atom catalysts. Synthesis and characterization of Pd1/NMCS using thermal transformation strategy. Credit: TIAN Zhengbin. Carbon ... "When nitrogen is readily available from nitrogen-fixing symbionts, the benefits of photoprotection apparently do not outweigh the costs of anthocyanin." Future of fall color Based on their electrons dot diagrams, what is the formula for the covalently bonded compound of nitrogen and hydrogen? 1 See answer Advertisement Advertisement aidanpedroza is waiting for your help. Add your answer and earn points. khansamueen khansamueen Answer: The main objective of a statistical mechanical calculation is drawing the phase diagram of a many-body system. In this respect, discrete systems offer the clear advantage over continuum systems of an easier enumeration of microstates, though at the cost of added abstraction. With this in mind, we examine a system of particles living on the vertices of the (biscribed) pentakis dodecahedron ...
N2O4 Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and Polarity. Dinitrogen tetroxide (N2O4) is commonly known as nitrogen tetroxide (NTO). It is colorless in solid form while in liquid and gaseous form; it has a characteristic reddish-brown color. It has an unpleasant, irritating acid-like smell.
The two letter N's in the N2 Lewis structure represent the nuclei (centers) of the nitrogen atoms. How many dots are needed to draw the Lewis dot diagram for nitrogen? A beryllium atom, with two valence electrons, would have the electron dot diagram below….Electron Dot Diagrams.
10+ Carbon Tetrachloride Lewis Structure. Carbon tetrachloride (ccl4) is a covalently bonded compound composed of a central carbon surrounded by 4 chlorine atoms in a tetrahedral structure. It is comprised of a single carbon (c) and four chlorine (cl) atoms. File:Carbon tetrachloride (flat).svg - Wikimedia Commons from upload.wikimedia.org It is…
1:51A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Diatomic Nitrogen).For the N2 ...Feb 4, 2021 · Uploaded by Wayne Breslyn
Lewis Structure of NO2. A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen. Let us look at the periodic table. Nitrogen belongs to group 15 ( or group 5) and has an atomic number of 7, therefore has a valency of 5. Oxygen belongs to group 16 ( or group 6) and has an atomic number of 8, therefore a valency of 6.
1:17A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For ...Sep 14, 2013 · Uploaded by Wayne Breslyn
Q. This is the correct dot diagram for sodium, group 1. Q. This is the correct dot diagram for nitrogen, group 15. Q. This is a correct dot diagram for neon, group 18. Q. This could be the dot diagram of. Mg, group 2.
Describe the electron dot diagram system of representing structure. Draw electron dot diagrams for ... nitrogen, 1 s 2 2 s 2 2 p 3, 5 valence electrons.Nitrogen: 1 s 2 2 s 2 2 p 3Lithium: 1 s 2 2 s 1Neon: 1 s 2 2 s 2 2 p 6Beryllium: 1 s 2 2 s 2
Explanation with diagram. Resonance in H 3 PO 3; This is a structure that can be shown using resonance structures. Explanation with diagram. Resonance in Dinitrogen I oxide (N 2 O) Resonance in Nitrogen IV oxide (NO 2) Resonance in dintrogen V oxide (N 2 O 5) Resonance in; Examples of Resonance in ions. Resonance in nitrate ion (NO 3 -)
1:39A step-by-step explanation of how to draw the NI3 Lewis Dot Structure (Nitrogen Triiodide).For the NI3 ...May 11, 2013 · Uploaded by Wayne Breslyn
Alternate lewis dot structure of water. 10+ Nh3 Lewis Structure. This is the reason why ammonia acts as a lewis base, as it can donate those electrons. (1) you have two chlorine atoms. Bonding and structure in covalent compounds. Electron dot structures or lewis dot formula can be drawn if the molecular formula of the compound is known.
1:31A step-by-step explanation of how to draw the N3- Lewis Dot Structure. For the N3- Lewis structure use the ...Aug 23, 2018 · Uploaded by Wayne Breslyn
NH2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. NH2, as we can see, is a chemical composition of nitrogen and hydrogen atoms. However, this has several forms of existence as chemical entities. As a neutral compound, this is known to exist as a radical known as amino radical and therefore has the formula NH2.
1:21Note: Nitrogen is in Group 5 (sometimes called Group V or Group 15). Since it is in Group 5 it will have 5 ...Aug 29, 2013 · Uploaded by Wayne Breslyn
13+ H2 Lewis Structure. You have a total of 8 valence electrons available to fill the octets of oxygen and hydrogen. All valence electrons of the atoms in lewis structures must be shown. H2S Lewis Structure - How to Draw the Dot Structure for … from i.ytimg.com. Most lewis structures you encounter will be covalent bonds.
Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell. As per the molecule N2, it has two atoms of Nitrogen.
Formal Charge on all atoms in NH3. Ammonia consists of three atoms of hydrogen and one atom of nitrogen in its molecule. Nitrogen has 5 valance electrons having configuration 1s 2, 2s 2, 2p 3.Hydrogen has 1 valance electron having the configuration 1s 1.Nitrogen is the central atom in an ammonia molecule and combines with three hydrogen atoms via a covalent bond.
1:48A step-by-step explanation of how to draw the NF3 Lewis Dot Structure (Nitrogen trifluoride).For the NF3 ...May 10, 2013 · Uploaded by Wayne Breslyn
13+ Dot And Cross Diagram. An ionic compound is formed by massive numbers of positive and negative ions. The dot and cross products. A structural formula in which electrons are represented by dots; A dot diagram (also called an electron dot diagram, and a lewis structure) is a way to show the valence electrons that surround an element.
Nitrogen is in group 5 so it forms three covalent bonds. There are three shared spaces between the circles, so add a dot and cross to each one. This incomplete dot and cross diagram shows only the bonding pairs of electrons. Finally, add in the non-bonding outer electrons. Nitrogen atoms have five outer electrons.
When isolated from graphite, graphene's chemical structure looks like a honeycomb of pure carbon atoms. It's one of the thinnest materials on Earth, yet is 200 times stronger than steel.





0 Response to "43 dot diagram for nitrogen"
Post a Comment