41 pi acceptor mo diagram
6 Sept 2020 ... English: MO diagram for an octahedral transition metal complex with pi donor ligands. Overlaid on an MO diagram of a complex with sigma bonding ... 1: MO Diagrams of Pi Donor Ligands and Pi Acceptor Ligands. fig-ch01_patchfile_01.jpg Figure 1.11.2: Electron ...
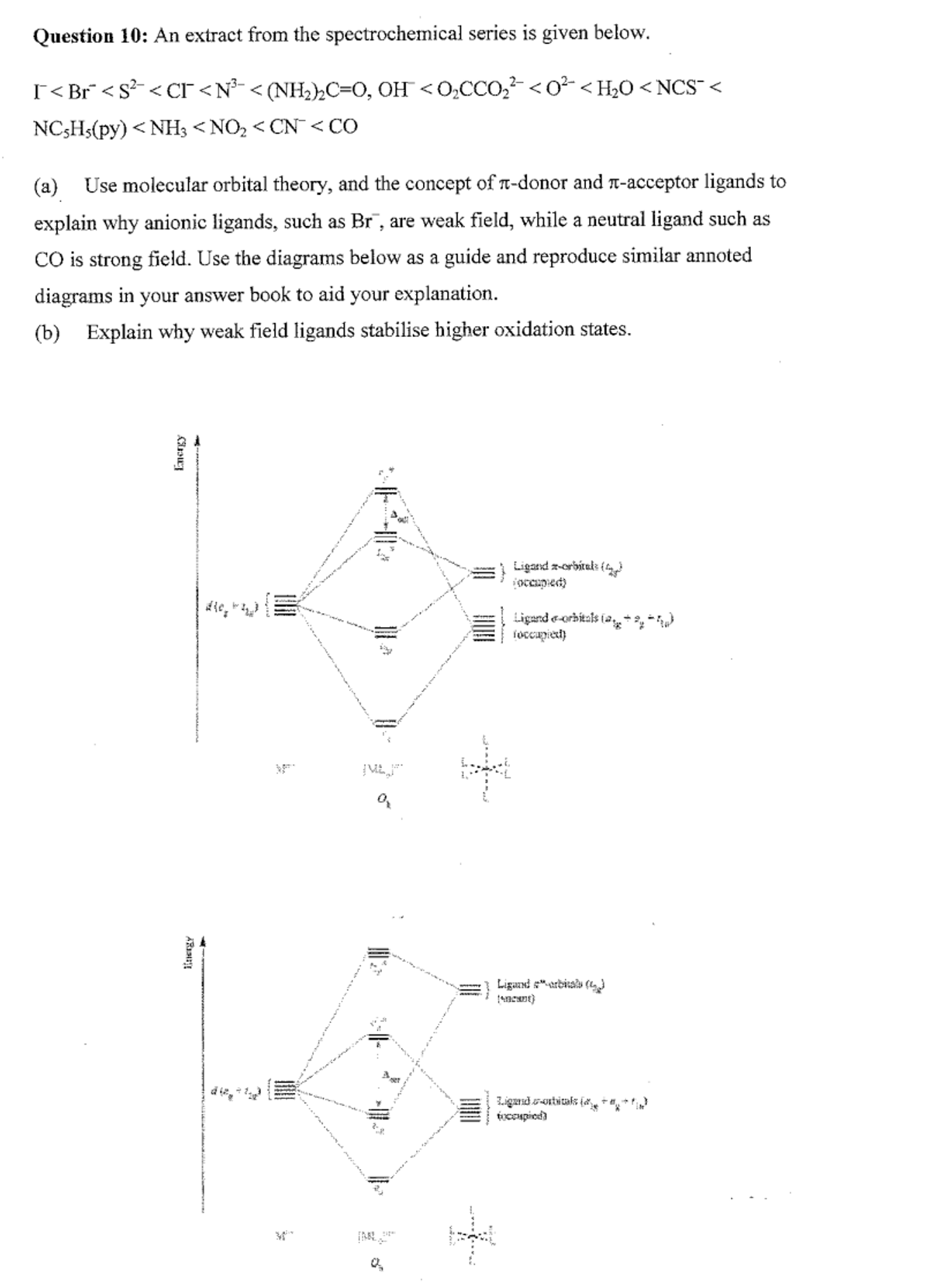
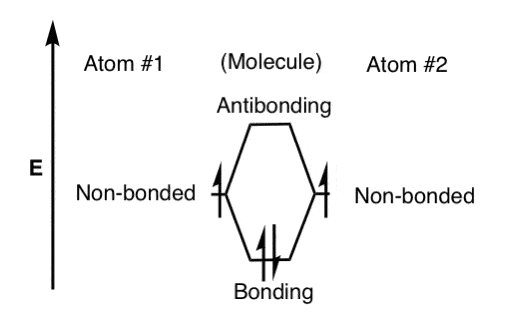
σ-only MO diagram for ML ... π interactions (π-donor/π-acceptor ligands) σ symmetry lone pairs ... Symmetry analysis for π orbitals (donors or acceptors).
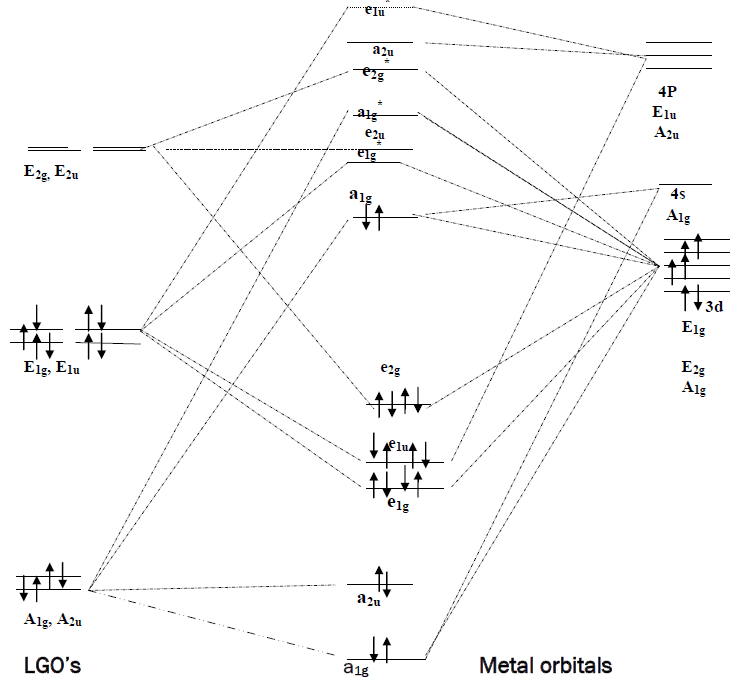
Pi acceptor mo diagram
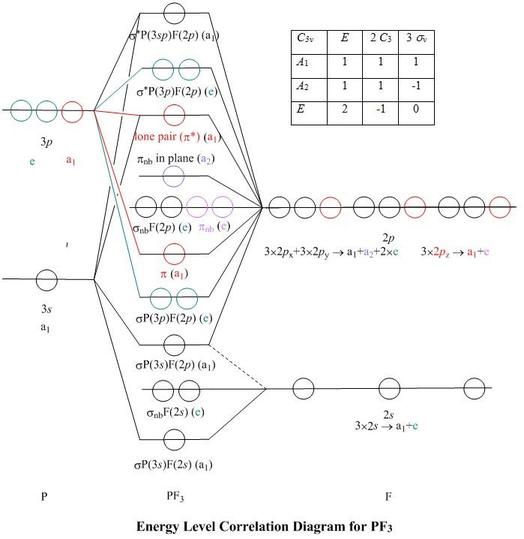
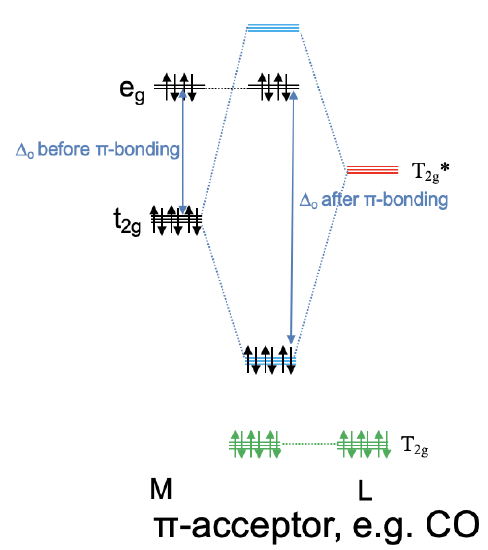
CO and other iso-electronic π-acceptor ligands. • CO ligands are very useful for characterising the amount of back donation in other ligands. Molybdenum disulfide belongs to a class of materials called 'transition metal dichalcogenides' (TMDCs). Materials in this class have the chemical formula MX 2, where M is a transition metal atom (groups 4-12 in the periodic table) and X is a chalcogen (group 16). The chemical formula of molybdenum disulfide is MoS 2. The crystal structure of molybdenum disulfide (MoS 2) takes the form of a ... π backbonding, also called π backdonation, is a concept from chemistry in which electrons move from an atomic orbital on one atom to an appropriate symmetry ...
Pi acceptor mo diagram. With 133 pm, the ethylene C=C bond length is shorter than the C−C length in ethane with 154 pm. The double bond is also stronger, 636 kJ mol −1 versus 368 kJ mol −1 but not twice as much as the pi-bond is weaker than the sigma bond due to less effective pi-overlap. In an alternative representation, the double bond results from two overlapping sp 3 orbitals as in a bent bond. Ligands that are neither π-donor nor π-acceptor give a value of Δo somewhere in-between. The magnitude of Δo determines the electronic structure of d4-d7 metal ... 26.04.2017 · 1. Basicity Basics. Thankfully, even if you’re just getting started on amines, this subject shouldn’t really be that new to you. You’ve likely encountered the problem of evaluating how acidic certain molecules are – such as, for example, in the 5 key factors that affect acidity.. From that unit, you may recall that any factor which makes a molecule’s conjugate base more stable will ... 17.06.2020 · With the gradual intensity of global energy crisis, hydrogen (H 2) is one of the most sustainable and clean energies for replacing fossil fuel energy [1, 2].Reforming natural gas to produce H 2 not only consumes a large amount of natural resources but also produces undesired carbon dioxide, which causes greenhouse effect [3,4,5].Splitting water into H 2 and oxygen (O 2) was from more than …
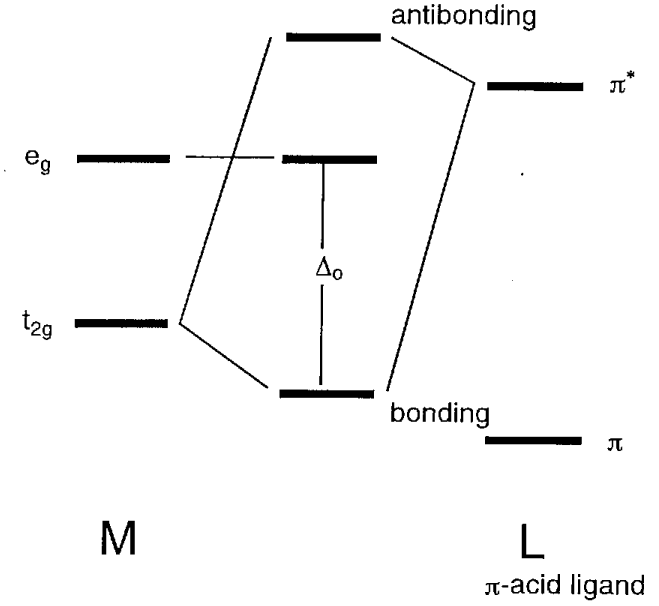
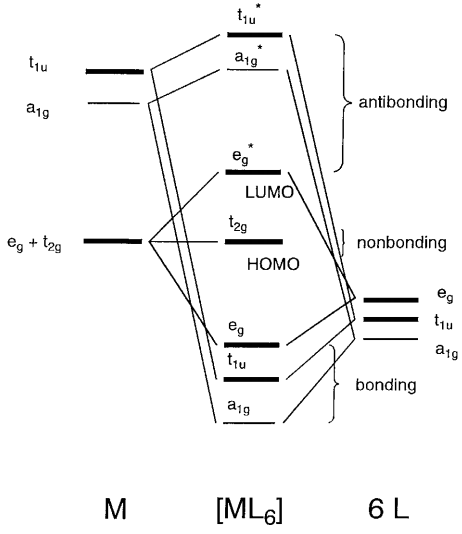
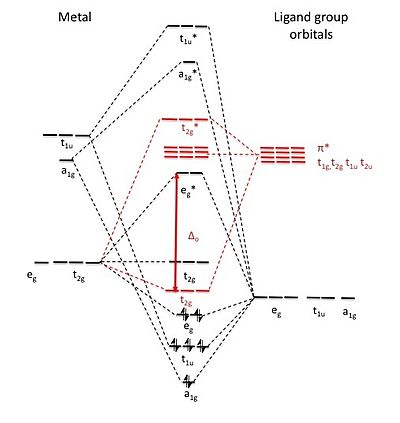
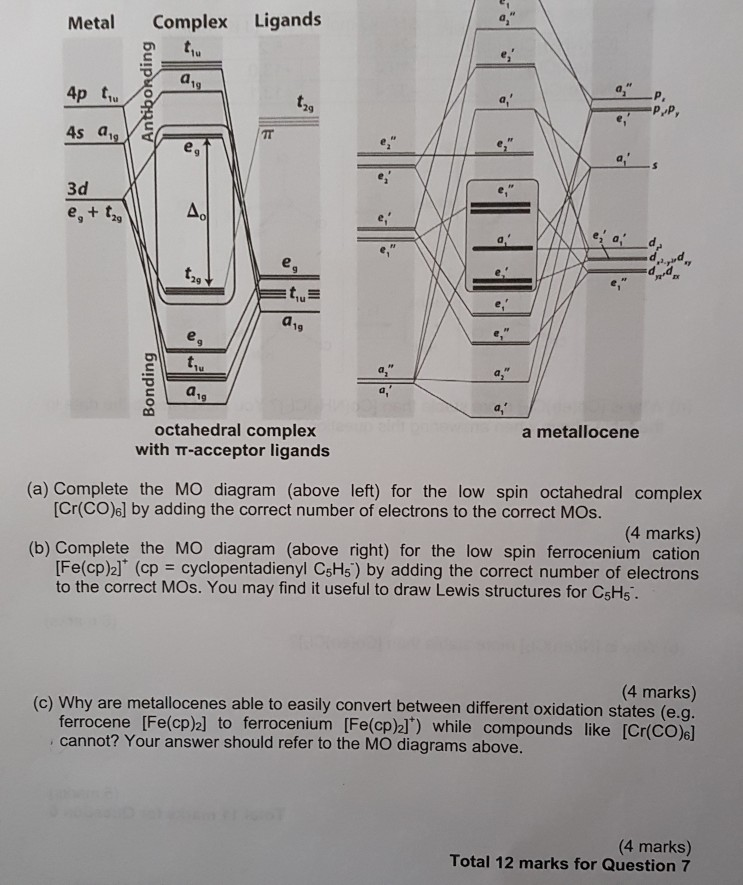
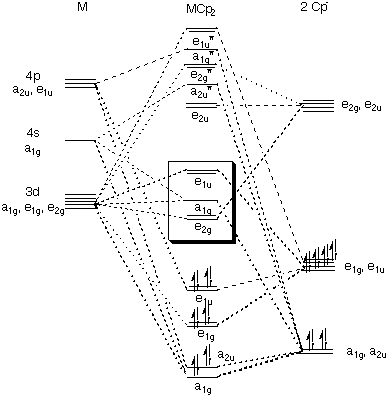
Academia.edu is a platform for academics to share research papers. ML6 molecular orbital energy diagrams incorporating π−acceptor and π−donor ligands. Relationship to spectrochemical series, and the trans-effect. The net result is that pi acceptor ligands (such as CO and N 2), with empty antibonding orbitals available to accept electrons from the metal, increase the size of D o. The spectrochemical series can be reconsidered with the possiblity of pi bonding in mind. It shows that the order (with some notable exceptions) goes as follows: three examples, this is because we have introduced only one π-acceptor or π- donor ligand. σ-donor ligands π∗-acceptor ligands. I- < Br- < S-2 ...
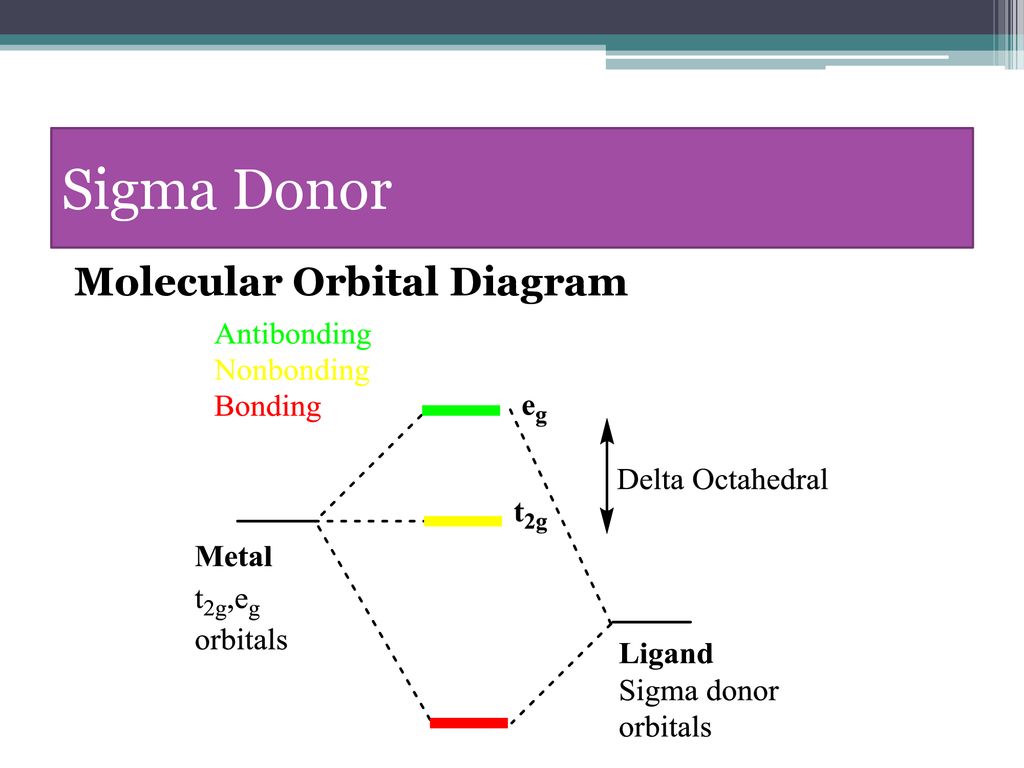
Hello everyone in this videos we will learn about the type of ligands Pi donor ligands, Pi acceptor ligands, How pi bond is formed means ... 16.07.2021 · The light-emitting properties of a new donor-acceptor type ... (MO) diagram for organo-transition ... luminescence and semiconductor properties of a quinacridone derivative with extended pi ... 28.02.2017 · The highest energy MO (pi 7) would have all p-orbital lobes alternating (6 nodes). It will resemble allyl and pentadienyl in that the second-lowest orbital (pi 2) will have a node right on the central carbon. In the pi 4 and pi 6 orbitals there will also be a node on the central carbon, in addition to additional nodes. π-donors. • π-acceptors. 2.1 MO Theory: σ-Donor Ligands. These ligands donate two e–s from an orbital of σ-symmetry. Examples include: H-, CH3.
CMOS DIGITAL INTEGRATED CIRCUITS BY SUNG MO KANG & YUSUF LEBLEBICI(prince367) Chaitanya Reddy. Download PDF. Download Full PDF Package. This paper. A short summary of this paper. 37 Full PDFs related to this paper. READ PAPER. CMOS DIGITAL INTEGRATED CIRCUITS BY SUNG MO KANG & YUSUF LEBLEBICI(prince367) Download. CMOS DIGITAL INTEGRATED …
Doping a semiconductor in a good crystal introduces allowed energy states within the band gap, but very close to the energy band that corresponds to the dopant type.In other words, electron donor impurities create states near the conduction band while electron acceptor impurities create states near the valence band. The gap between these energy states and the nearest energy band is usually ...
π backbonding, also called π backdonation, is a concept from chemistry in which electrons move from an atomic orbital on one atom to an appropriate symmetry ...
Molybdenum disulfide belongs to a class of materials called 'transition metal dichalcogenides' (TMDCs). Materials in this class have the chemical formula MX 2, where M is a transition metal atom (groups 4-12 in the periodic table) and X is a chalcogen (group 16). The chemical formula of molybdenum disulfide is MoS 2. The crystal structure of molybdenum disulfide (MoS 2) takes the form of a ...
CO and other iso-electronic π-acceptor ligands. • CO ligands are very useful for characterising the amount of back donation in other ligands.

Why Do The Halides Run In Reverse Orders In The Spectrochemical Series And The Trans Effect Series Chemistry Stack Exchange

How To Rationalise With Mo Theory That Co Is A Two Electron Donor Through Carbon Chemistry Stack Exchange















0 Response to "41 pi acceptor mo diagram"
Post a Comment