44 oh- molecular orbital diagram
I.G Molecular Orbital Theory | ScienceDirect Topics The molecular orbital diagram for even a simple octahedral complex looks complicated ( Figure 5 ), but most of the time the information needed may be obtained by just considering the A sophisticated version of this diagrammatic scheme remains in use even today as a qualitative predictive device. Molecular Orbital Theory Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the The bonding molecular orbital concentrates electrons in the region directly between the two nuclei. The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both...
Figure 2. Energy level diagram for the molecular orbitals of OH ). H... Carbon Dioxide, Orbit and Frontier | ResearchGate, the professional network for scientists. The kinetics of the reactions of water, hydroxide ion and sulfide species with CO2, OCS and CS2 are investigated using the molecular orbital approach and available kinetic data.
Oh- molecular orbital diagram
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described... Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the...
Oh- molecular orbital diagram. PDF Lecture 1 Lecture 6: ML6 molecular orbital energy diagrams incorporating p-acceptor and p-donor ligands. Electron counting revisited and link to spectrochemical Using group theory it is possible to determine the symmetry of the orbitals involved. i) determine the point group of the molecule (in this case Oh)... PDF PowerPoint Presentation u Stable electronic configurations: MO Energy Level Diagrams Reviewed u Electron count preference u Electron count and Oxidation States u Ligands. • Carbon Monoxide • Phosphines • Cyclopentadienide and arenes • Hydrides and dihydrogen. 8.4 Molecular Orbital Theory - Chemistry Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. Figure 8. This is the molecular orbital diagram for the homonuclear diatomic Be2+, showing the molecular orbitals of the valence shell only. H3O+ Lewis Structure, Geometry, Hybridization, and MO Diagram Mar 13, 2022 · H3O+ Molecular Orbital (MO) Diagram. A molecular orbital diagram of any molecule gives us an idea about the mixing of orbitals in the molecule. Talking about the overlap diagram of H3O+, it is almost similar to H2O but with one electron less and one hydrogen more. Given below is the image of the molecular orbital diagram of H3O+ and also that ...
Hydroxylation and molecular adsorption behavior of SnO2 ... The orbital changes are obvious due to the dissociation of molecules. Fig.11 (b) is the DOS diagram of O 2 and H 2 O molecules adsorbed on the hydroxylated SnO 2 (110) crystal plane (OHSnO 2 ). The two adsorptions increase and decrease the DOS at the Fermi level respectively, and the corresponding conductivity of the crystal plane also ... Molecular orbital diagram of HF and OH- - YouTube #MOT #BMO #ABMO #HF #CO #NO #CN #OHHello everyoneThis is shivam here To follow me on instagram search - Sshivam898To join telegram group click on the given... Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory. Valence Bond Theory proposes that electrons are localized between two atoms. MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available
PDF Molecular | 90" (porbitals). This dilemma has been resolved by orbital MOLECULAR ORBITAL and valence bond calculations of the w-electron energies of unsaturated molecules custom-arily start with models in which lecular orbital representations. . Knowledge of how to s e t up a n atomic orbital model for an organic molecule i s c r u c i a l to the LCAO... Molecular Orbital Theory: Energy level diagram for molecular orbitals Molecular orbital theory was put forward by Hund and Mullikan in 1932. This theory is modern and more rational. This theory assume that in molecules, atomic orbitals lose their identity and the electrons in molecules are present in new orbitals called molecular orbitals. What is the molecular orbital diagram for oxygen? - Quora The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital. techiescientist.com › hno3-lewis-structureHNO3 Lewis Structure, Molecular Geometry, Hybridization, and ... Mar 12, 2022 · MO Diagram of HNO3. The sigma bonds between N and O atoms are formed by 2sp2 orbital of nitrogen and a hybrid orbital atom from O atoms. As a result, three sigma bonding and antibonding orbitals are formed. The single sigma bond between hydrogen and oxygen use 1s orbital of hydrogen 2sp3 orbitals of oxygen.
8.2 Hybrid Atomic Orbitals – Chemistry This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl 2 molecule. Each of the two sp hybrid orbitals holds one electron and is thus half filled and available for bonding via overlap with a Cl 3 p orbital.
Molecular Orbitals: Molecular Orbital Theory | SparkNotes Molecular orbital theory posits the notion that electrons in molecules likewise exist in different orbitals that give the probability of finding the electron at Notice that the orbitals of the separated atoms are written on either side of the diagram as horizontal lines at heights denoting their relative energies.
PDF Microsoft PowerPoint - Polyatomic Molecular Orbital Theory... Polyatomic Molecular Orbital Theory. Transformational properties of atomic orbitals. Oh A1g x2+y2+z2 Eg (2z2-x2-y2, x2-y2) T1g (Rx,Ry,Rz) T2g (xz, yz, xy) T1u (x,y,z) … MO diagram of homonuclear diatomic molecules.
Asked for: molecular orbital energy-level diagram, valence electron... A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Use a molecular orbital energy-level diagram to predict the valence-electron configuration and bond order of the H 2 2 − ion. Is this a stable species?
NCERT Solutions for Class 11 Chemistry Chapter 4 ... - VEDANTU NCERT Exercise: 1. Explain the formation of a chemical bond. Ans: A chemical bond is defined as an attractive force that holds the constituents (atoms, ions, etc.) together in a chemical species. Various theories have been suggested for the formation of chemical bonds such as the electronic theory, valence shell electron pair repulsion theory, valence bond theory, and molecular …
An introduction to molecular orbital theory The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative calculations, including those that Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate...
Molecular Orbitals of The Allyl Cation, Allyl Radical, and ... The lowest-energy molecular orbital had all the phases in the contributing p-orbitals aligned the same way. In other words, there were no nodes between Drawing out the molecular orbitals for a single pi bond seems simple enough. But what happens to the molecular orbital diagram if we add a third...
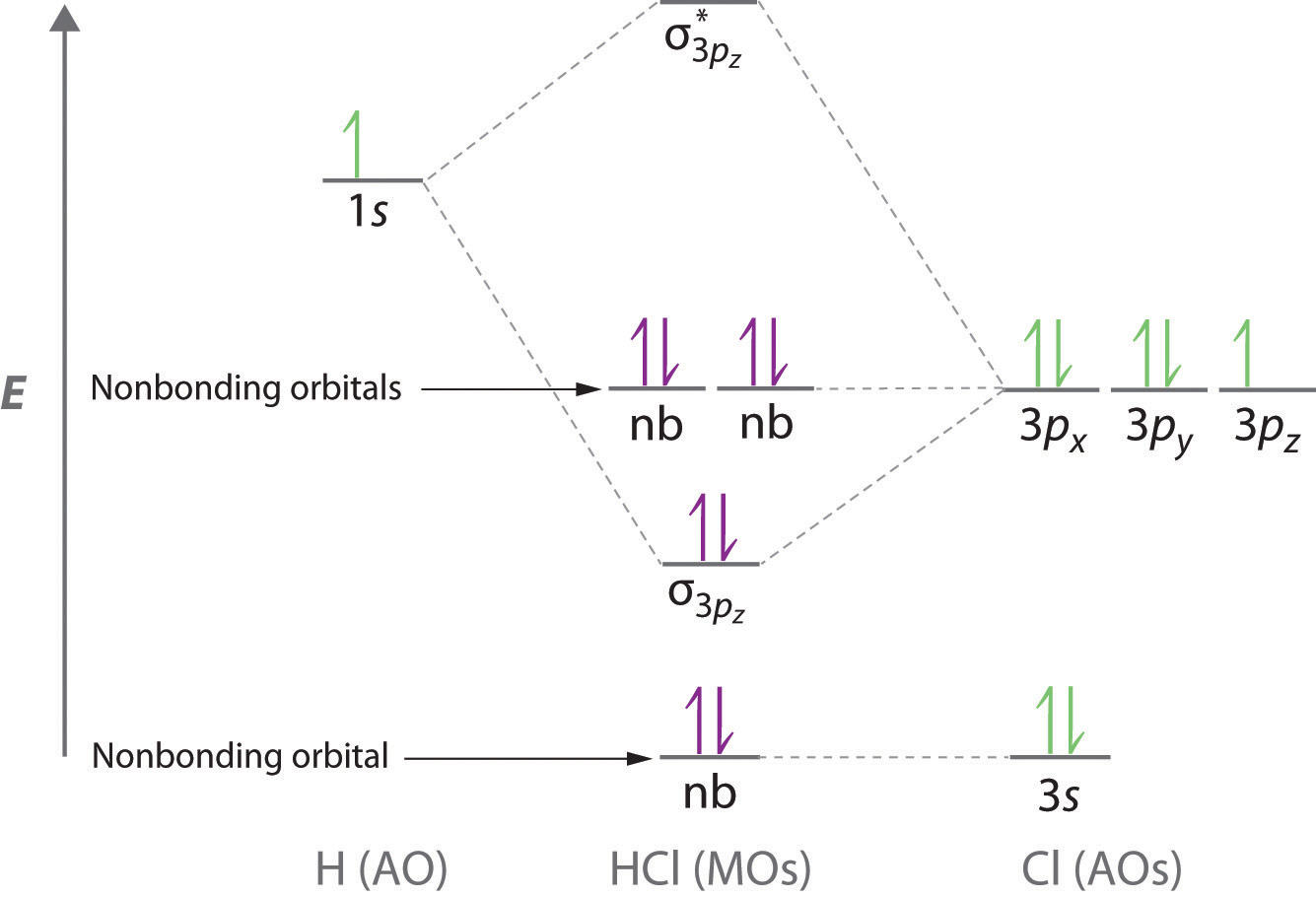
Molecular orbital diagram of hydroxide ion? - Chemistry Stack... I would like to understand how to create a molecular orbital diagram for the hydroxide ion from scratch. This includes understanding the shape of the molecular orbital. Once I can understand that, hopefully I will be able to visualise the HOMO of the OH- ion.
Molecular cloud - Wikipedia A molecular cloud, sometimes called a stellar nursery (if star formation is occurring within), is a type of interstellar cloud, the density and size of which permit absorption nebulae, the formation of molecules (most commonly molecular hydrogen, H 2), and the formation of H II regions.This is in contrast to other areas of the interstellar medium that contain predominantly ionized gas.
H2S Lewis Structure, Molecular Geometry, Hybridization ... 2 days ago · All these explain the molecular geometry of H2S. H2S Molecular Orbital (MO) Diagram. The molecular orbital diagram of H2S can be explained in the following way. This is the MO diagram of H2S. The left-hand side will contain the atomic orbitals of sulfur i.e 3s2 3px2 3py1 3pz1. And on the right-hand side, there will be atomic orbitals of hydrogen.
Molecular orbital diagrams - Overleaf, Online LaTeX Editor Molecular orbital diagrams provide qualitative information about the structure and stability of the electrons in a molecule. This article explains how to create molecular orbital diagrams in LaTeX by means of the package MOdiagram. For information about the more traditional molecular structure...
Molecular Orbital Theory: Explanation, Illustrations and... - Embibe Molecular Orbital Theory: It is used to define the bonding in molecules which cannot be explained with the help of Valence Bond Theory. Molecular Orbital Theory: To simplify things, we will consider the interaction of the orbitals containing valence electrons to create molecular orbitals.
Chapter 4 - Chemical Bonding and Molecular Structure ... Each chlorine atom has a single 3p orbital that is completely occupied. The overlap of a phosphorus sp 3 hybrid orbital with a singly occupied chlorine 3p orbital results in the formation of P–Cl bonds. Three lone pairs are held by each Cl atom. 2. Using molecular orbital theory, compare the bond energy and magnetic character of O 2 + and O 2 ...
Polyatomic Species | Molecular Orbital Theory | Chemogenesis Molecular orbital calculations using software such as Spartan or Gaussian are performed by assigning the five nuclei positions in space, and then The σ skeleton of an organic molecule is described using hybridized-VSEPR atoms with the Hückel π-system functional groups superimposed 'on top'.
Singlet oxygen - Wikipedia Molecular orbital diagram of two singlet excited states as well as the triplet ground state of molecular dioxygen. From left to right, the diagrams are for: 1 Δ g singlet oxygen (first excited state), 1 Σ + g singlet oxygen (second excited state), and 3 Σ − g triplet oxygen (ground state). The lowest energy 1s molecular orbitals are ...
Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep The molecular orbital diagram representing this order of energy levels is shown in fig. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value is negative or zero, it means that the molecule is unstable.
Molecular Orbital Theory : chemhelp Molecular Orbital Theory. I'm having a lot of trouble with this stuff. I don't really know how to start these questions (such as how to draw So here, I basically ask, how do I draw a correlation diagram? Like, how do I know how many electrons to put in the bonding atomic orbital and antibonding atomic orbital?
Energy level diagram for Molecular orbitals - Chemical Bonding and... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons. 1) If Nb > Na ,the molecule is stable because greater number of bonding orbitals are occupied than antibonding orbital, resulting in a net force of attraction.
atom | Definition, Structure, History, Examples, Diagram ... Feb 03, 2022 · atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element. As such, the atom is the basic building block of chemistry. Most of the atom is empty space. The rest consists of a positively charged nucleus of protons and neutrons surrounded by a ...
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... - Molecular orbital are formed by addition and subtraction of AO's. Æ Linear Combination of Atomic Orbitals (LCAO). - like hybrid AO's but the • Energy level diagram represents this interaction. - Two s orbitals interaction to create a low energy bonding and high energy anti-bonding molecular orbital.
Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
Molecular Orbital diagram of NO(nitric oxide) molecule Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12.
Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the...
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described...

0 Response to "44 oh- molecular orbital diagram"
Post a Comment