40 orbital diagram for nitrogen
20.02.2021 · Orbital Diagram For Carbon (C) | Carbon Electron Configuration. February 20, 2021 by Sneha Leave a Comment. Carbon Electron Configuration: If you guys have come across our recent article then it would be easy for you all to understand the concept. But if you are new here and looking for the information related to the carbon element and its electronic … The simplest kind of orbital is the s orbital, which is spherical in shape, with the nucleus of the atom at its centre. Other orbitals include the p , d and f orbitals. For historical reasons the designated letters stand for sharp , principal , diffuse , and fundamental , based on the spectral line observations associated with them, but the association is no longer of any significance.
Diagram of Body Description Adapted Function; Anguiliform (eel shape) Maneuvering in crevasses: Fusiform (bullet, or torpedo shape) Lowering frictional resistance in fast swimmers: Depressiform (broad shape and flat top to bottom) Lying on or below the surface of the sand: Compressiform (tall, thin shape and flat side to side) Entering vertical ...

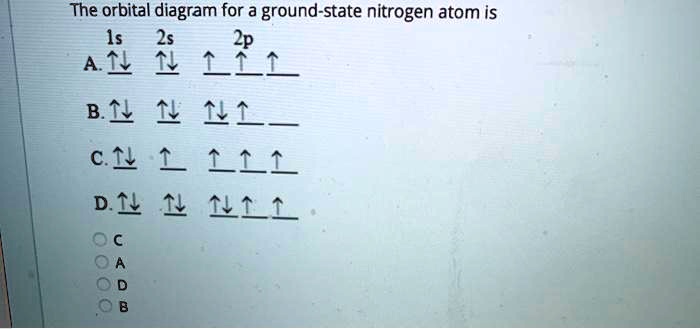
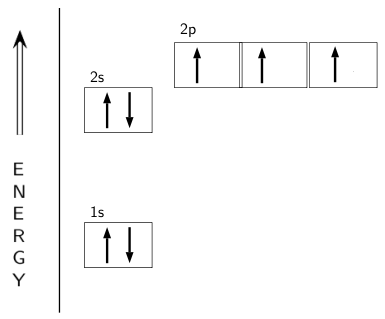
Orbital diagram for nitrogen
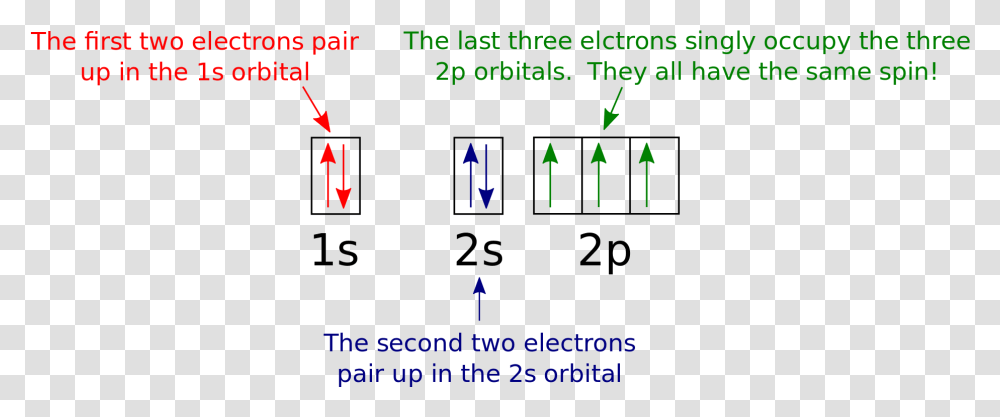
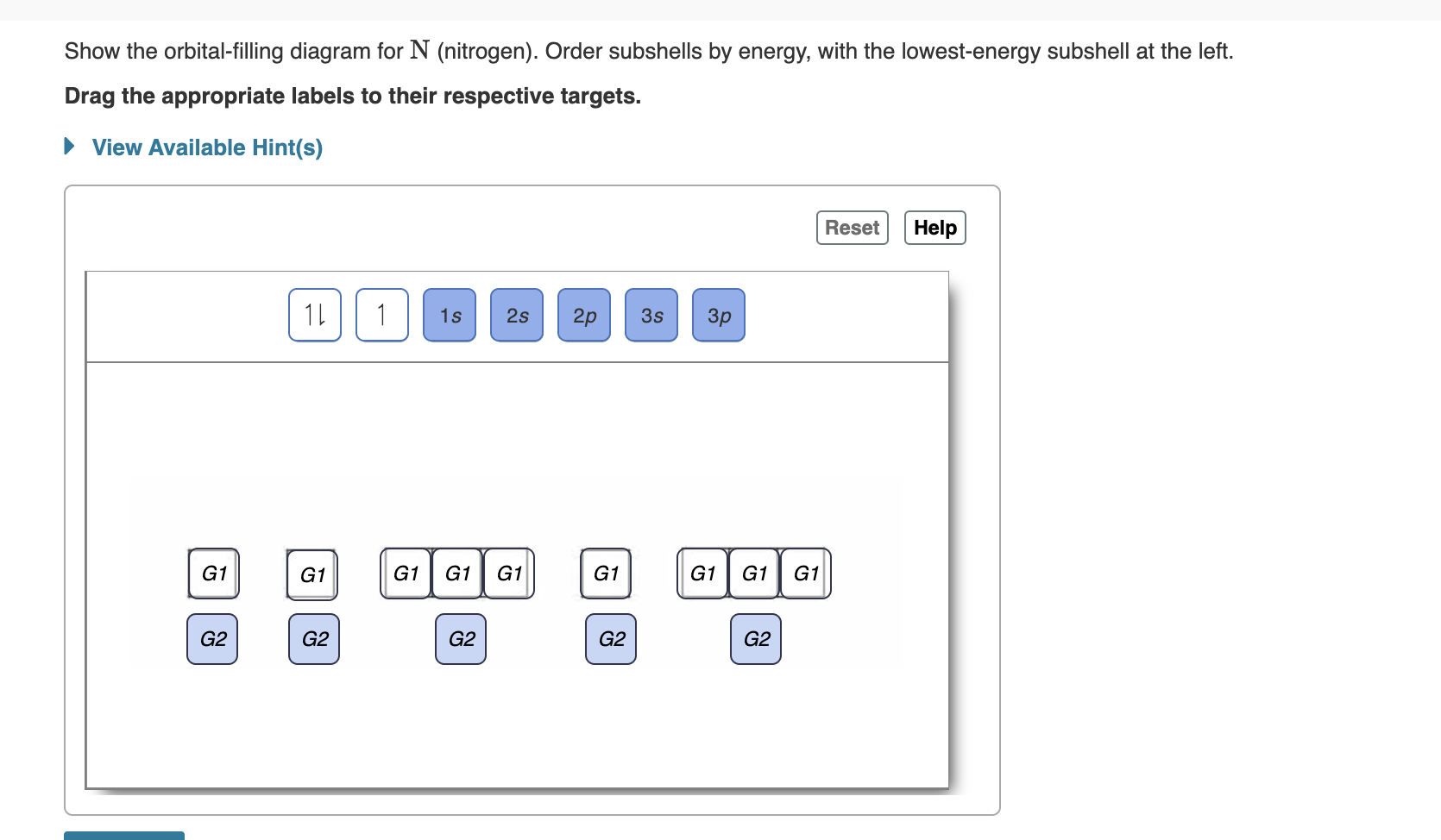
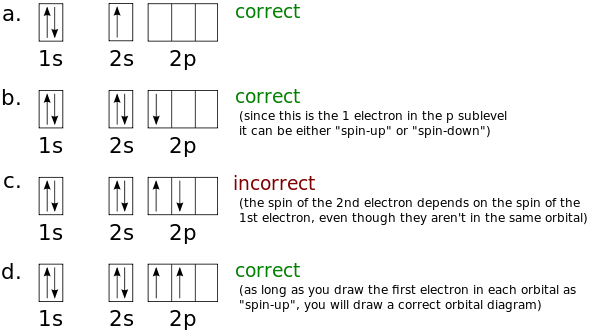
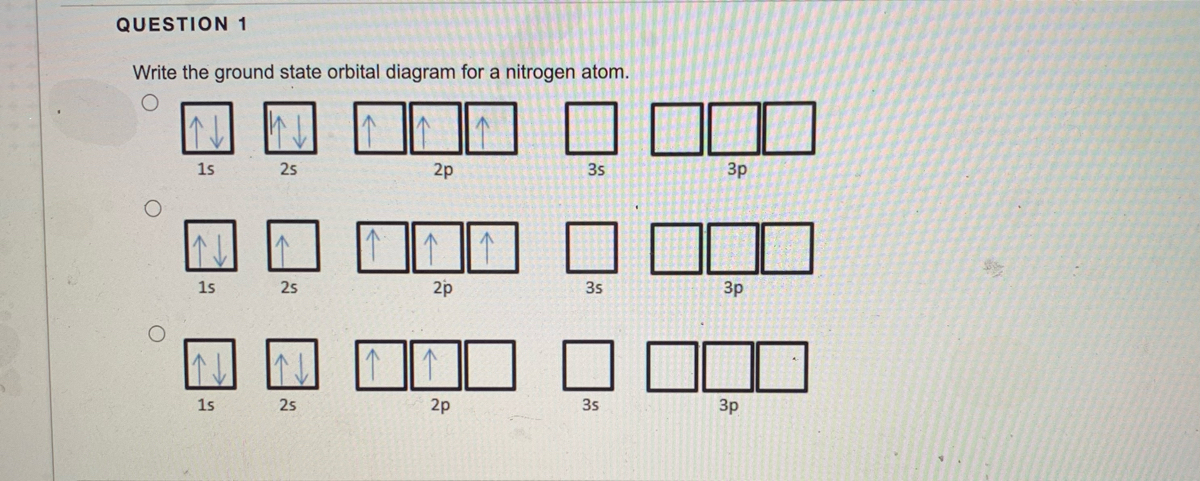
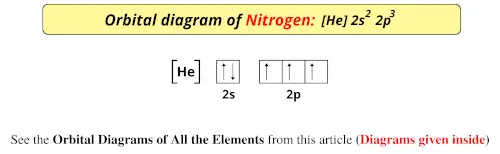
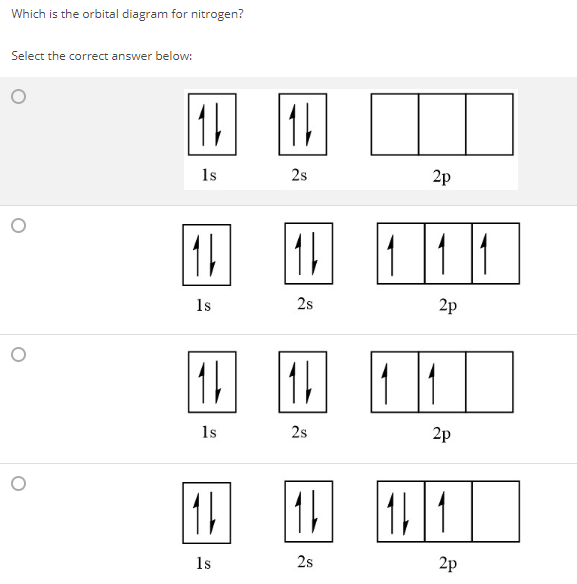
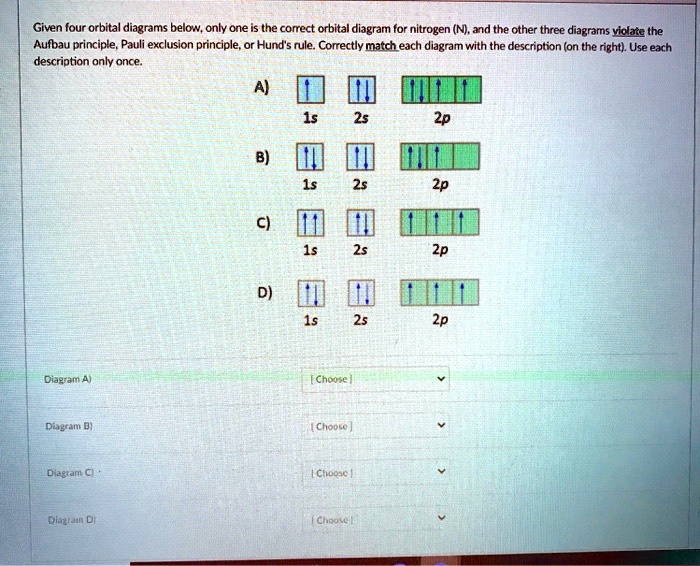
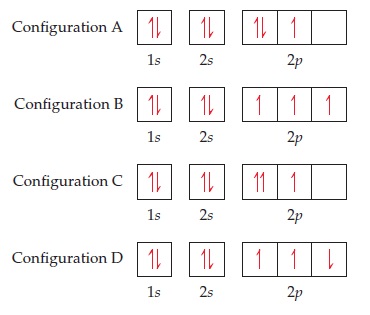
23.02.2016 · In the same way, the orbital filling diagram for nitrogen will be: It’s not until we reach oxygen, where the electrons will start to double up, just because there’s no alternative: And that’s the basic idea behind orbital filling diagrams! One big example to give you an idea of what a big one looks like . Now for the pain of doing the orbital filling diagram of lead: 1s 2 2s 2 2p … 2p. To figure out the configuration on your own, you can follow the orbital diagram to map out which shells will be filled first. According to Hund’s rule, electrons fill all orbitals of equal energy with one electron before pairing electrons. That means that for carbon, the two electrons in the 2p subshell would not occupy the same orbital ... The following molecular orbital diagram may be used for the following problems. For oxygen and fluorine, the σ 2p orbital should be lower in energy than the π 2p. However, the diagram will still yield correct bond order and magnetic behavior for these molecules. ____ 29. According to molecular orbital theory, which of the followin g species is the most likely to exist? a. H 2 2-b. …
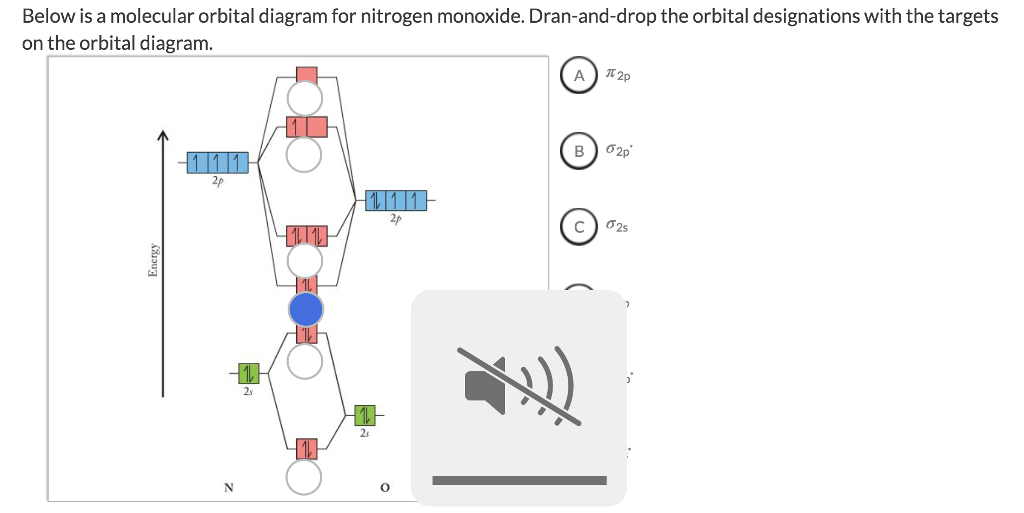
Orbital diagram for nitrogen. In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory.For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three ... Figure 1.5 is a schematic diagram of these attractive and repulsive forces. It provides a starting point for our discussion of bonding. Figure 1.5. Bonding Forces in a Hydrogen Molecule. When a covalent bond forms between two hydrogen atoms, there are two sets of electrostatic repulsions (nuclear–nuclear and electron–electron, red), but four sets of electrostatic attractions (green). … Description. The sun, which drives the water cycle, heats water in the ocean and seas. Water evaporates as water vapor into the air.Some ice and snow sublimates directly into water vapor. Evapotranspiration is water transpired from plants and evaporated from the soil. The water molecule H 2 O has smaller molecular mass than the major components of the atmosphere, … 14.03.2019 · Molybdenum disulfide is naturally inert for alkaline hydrogen evolution catalysis, due to its unfavorable water adsorption and dissociation feature originated from the …
The following molecular orbital diagram may be used for the following problems. For oxygen and fluorine, the σ 2p orbital should be lower in energy than the π 2p. However, the diagram will still yield correct bond order and magnetic behavior for these molecules. ____ 29. According to molecular orbital theory, which of the followin g species is the most likely to exist? a. H 2 2-b. … 2p. To figure out the configuration on your own, you can follow the orbital diagram to map out which shells will be filled first. According to Hund’s rule, electrons fill all orbitals of equal energy with one electron before pairing electrons. That means that for carbon, the two electrons in the 2p subshell would not occupy the same orbital ... 23.02.2016 · In the same way, the orbital filling diagram for nitrogen will be: It’s not until we reach oxygen, where the electrons will start to double up, just because there’s no alternative: And that’s the basic idea behind orbital filling diagrams! One big example to give you an idea of what a big one looks like . Now for the pain of doing the orbital filling diagram of lead: 1s 2 2s 2 2p …












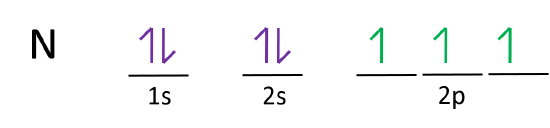












0 Response to "40 orbital diagram for nitrogen"
Post a Comment