40 lewis dot diagram of nitrogen
2 days ago · Construction of NO3 Lewis Dot Structure. 1. In the ion NO3, there is 1 atom of nitrogen and 3 atoms of oxygen. It also has one negative charge. 2. Nitrogen and oxygen belong to periods 5A and 6A groups respectively in the periodic table. Hence, oxygen has 6 and nitrogen has 5 valence electrons in their outer shell. 3. Notice the number of ... Lewis Electron Dot Diagram For Nitrogen, Nitrogen Facts, Symbol, Discovery, Properties, Uses, Lewis Dot Structure for Nitrogen Atom (N) YouTube, How to Draw the Lewis Dot Structure for N2: Nitrogen Gas, NF3 Lewis Structure How to Draw the Dot Structure for
Lewis dot diagram for c2h2. + • Bright spot Schematic diagram Passing through magnetic field applied perpendicular to the path of the cathode rays Deflection perpendicular to the applied magnetic field Cathode Anode S Fluorescent material (ZnS) N - + H.V. Schematic diagram • Bright spot The above experiments were carried out with ...

Lewis dot diagram of nitrogen
NO2 Lewis Dot Structure. Whenever something burns in the air, Nitrogen oxides will be formed. The reason for this is that the air we breathe mainly consists of Nitrogen (78%) and Oxygen (21%), and these combine in the presence of energy (from burning materials) in the environment. The nitrogen is the central atom and there is one lone pair on it. The lewis structure of NH2OH has a total of 3 lone pairs and 4 bond pairs. NH2OH lewis structure is drawn with the same procedure as the NH2Cl lewis structure. Follow some steps for drawing the lewis dot structure for NH2OH 1. Count total valence electron in NH2OH How is the Lewis structure for nitrogen dioxide drawn? NO2 has 5+6+6=17 valence electrons. N is in the center and sp2 hybridized to a triangular planar shape. One O atom is double bonded and has two nonbonded electron pairs on it. The other O atom is single bonded with three nonbonded electron pairs. There is a single electron on the N atom.
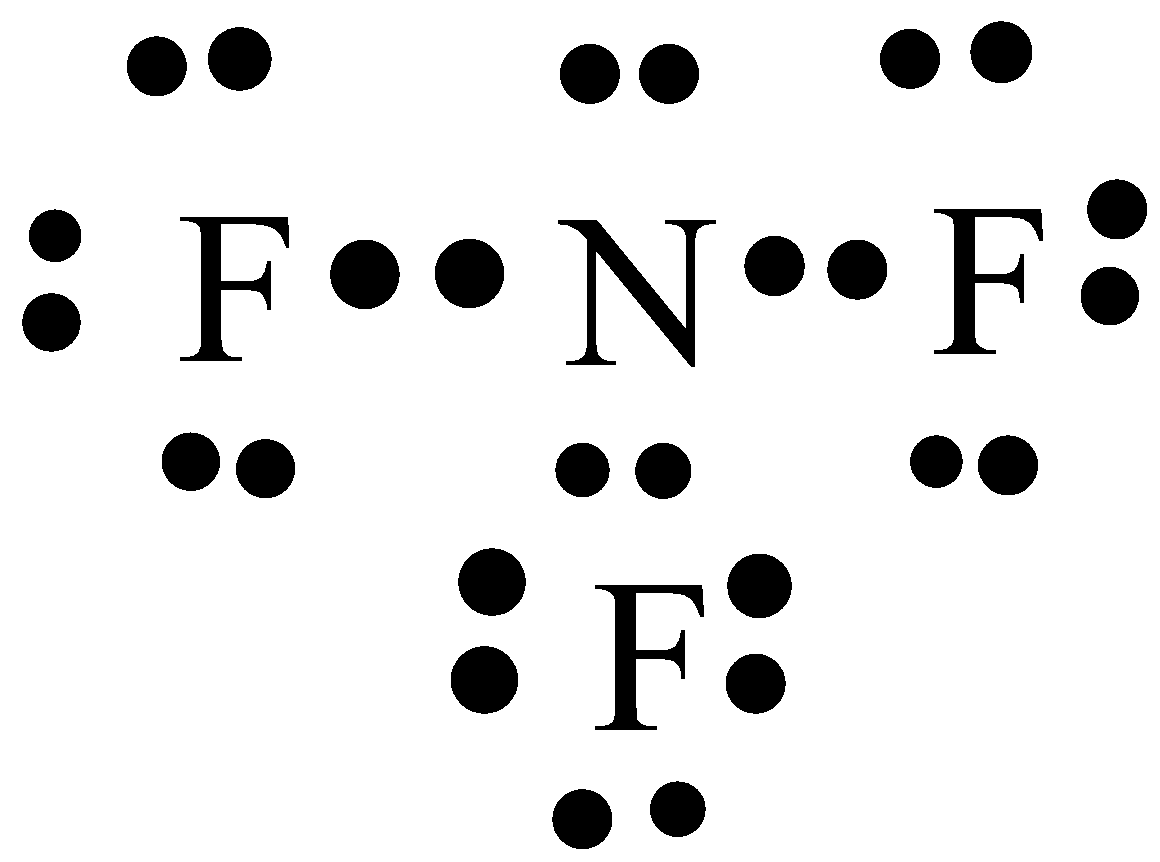
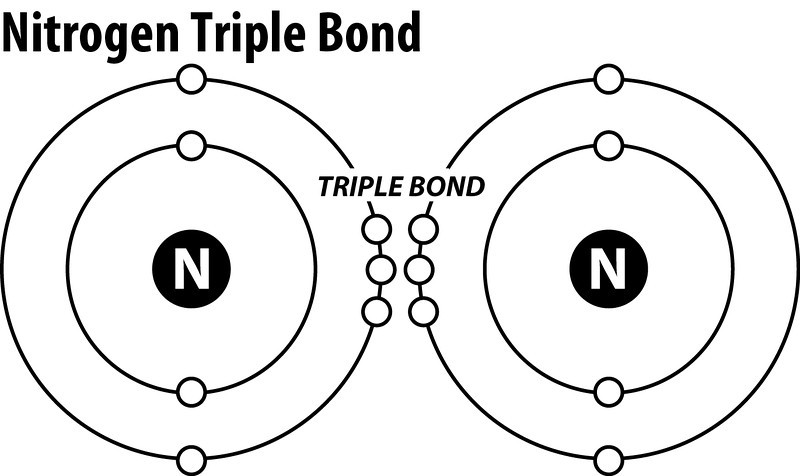
Lewis dot diagram of nitrogen. • (3c) ·Lewis dot diagrams are used to represent valence electrons in an element. Structural formulas show the arrangements of atoms and bonds in a molecule and are represented by Lewis dot structures. • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding. In the lewis structure of Nitrogen trifluoride (NF 3), there are three N-F bonds and one lone pair on nitrogen atom. Each fluorine atom has three lone pairs. Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps. What is the Lewis dot structure for nitrogen gas? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable. The two letter N's in the N2 Lewis structure represent the nuclei (centers) of the ... It's easiest to think in terms of dots to make the N 2 Lewis structure. Nitrogen needs to bond three times, shown as the lone dots on the left, right and bottom of the N atoms in the below diagram. There is also a pair of dots, representing two more electrons, that won't bond, on top of each N.
Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules. 3. Determine the electron and molecular geometry of the produced molecules. Background: Scientists often create models to represent either a physical or ... A Lewis base is an atomic or molecular species where the highest occupied molecular orbital (HOMO) is highly localized. Typical Lewis bases are conventional amines such as ammonia and alkyl amines. Other common Lewis bases include pyridine and its derivatives. Some of the main classes of Lewis bases are (b) Nitrogen has three vacancies in its Lewis dot diagram. (c) Carbon has four vacancies in its Lewis dot diagram. (d) Phosphorus has three vacancies in its Lewis dot diagram. 5. (a) Xenon is a nonmetal. (b) Copper is a metal. (c) Manganese is a metal. (d) Carbon is a nonmetal. 7. In metallic bonding, the electrons exist in a sea of electrons. Lewis dot structure is a diagram that represents the number of valence electrons of an element through dots around the element symbol. Learn about Lewis dots and understand single bonds, double ...
A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. Lewis diagram is a representation of how electrons are arranged around individual atoms in a structure. NCl3 lewis structure is the same as the NF3 structure. It contains one nitrogen atom at the center and three chlorine atoms spaced evenly around it. What is the Lewis dot diagram for nitrogen? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable.
The lewis structure of n2h2 shows c. each nitrogen has one nonbinding electron pair Lewis structures are diagrams that show the bonds between the atoms of a molecule and the lone pair of electrons that might exist in a molecule. Further Explanation. Lewis structures can be drawn for each covalently bonded molecule, as well as coordination ...
Note: The most important thing about the Lewis dot structure is that only valence electrons take part in chemical bonding. Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2.
beryllium and nitrogen lewis dot structure. Lewis Structures are important to learn because they help us predict: the shape of a molecule. Step 1: Determine the total number of electrons available for bonding. Nitrogen (IV) oxide (NO 2) is a well-known example. Electron dots are typically arranged in four pairs located on the four "sides" of ...
Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li
What is the dot structure of NO2? The NO2 Lewis structure has a total of 17 valence electrons. It's not common to have an odd number of valence electrons in a Lewis structure. Because of this we'll try to get as close to an octet as we can on the central Nitrogen (N) atom.
1 day ago · C2H4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Hydrocarbons form an essential and inseparable portion of the science of chemistry. Be it petroleum, crude oil, or natural gas, the majority of hydrocarbons are found naturally in these fossil fuels.
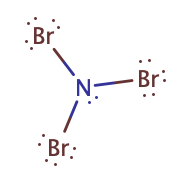
The element which repeats least in the compound should be the central atom in the lewis diagram. So, in the case of NBr3, the nitrogen atom repeated only one time whereas the bromine atom repeats three times. Hence, put the nitrogen atom at central position whereas spread the three bromine atom around it.
A step-by-step explanation of how to draw the Lewis dot structure for N (Nitrogen). I show you where Nitrogen is on the periodic table and how to determine ...
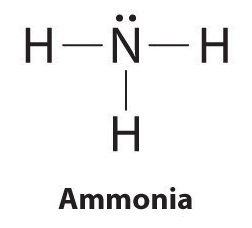
Lewis Structure (electron dot diagram) for ammonia OR Note that there are 3 covalent bonds (3 bonding pairs of electrons) in total, and that there is a lone pair (non-bonding pair) of electrons on the nitrogen atom.
Nitrogen appears as a colorless odorless gas. Noncombustible and nontoxic. Makes up the major portion of the atmosphere, but will not support life by itself. Used in food processing, in purging air conditioning and refrigeration systems, and in pressurizing aircraft tires.
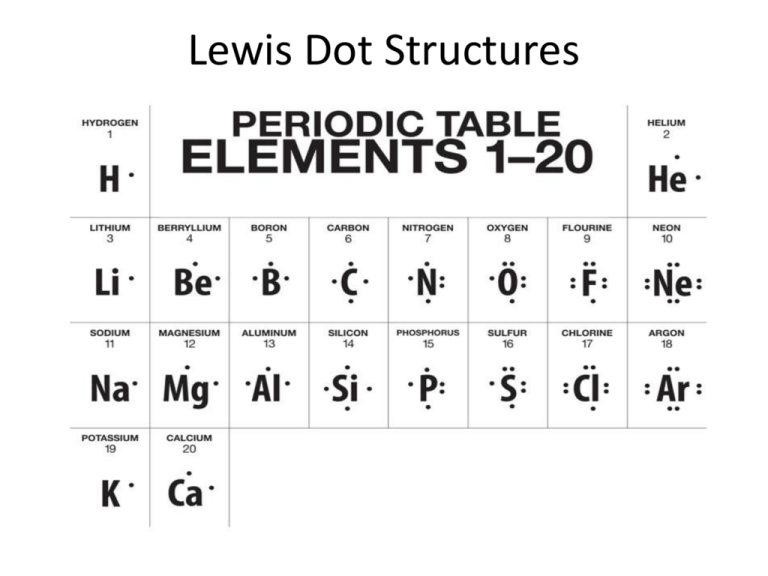
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below.
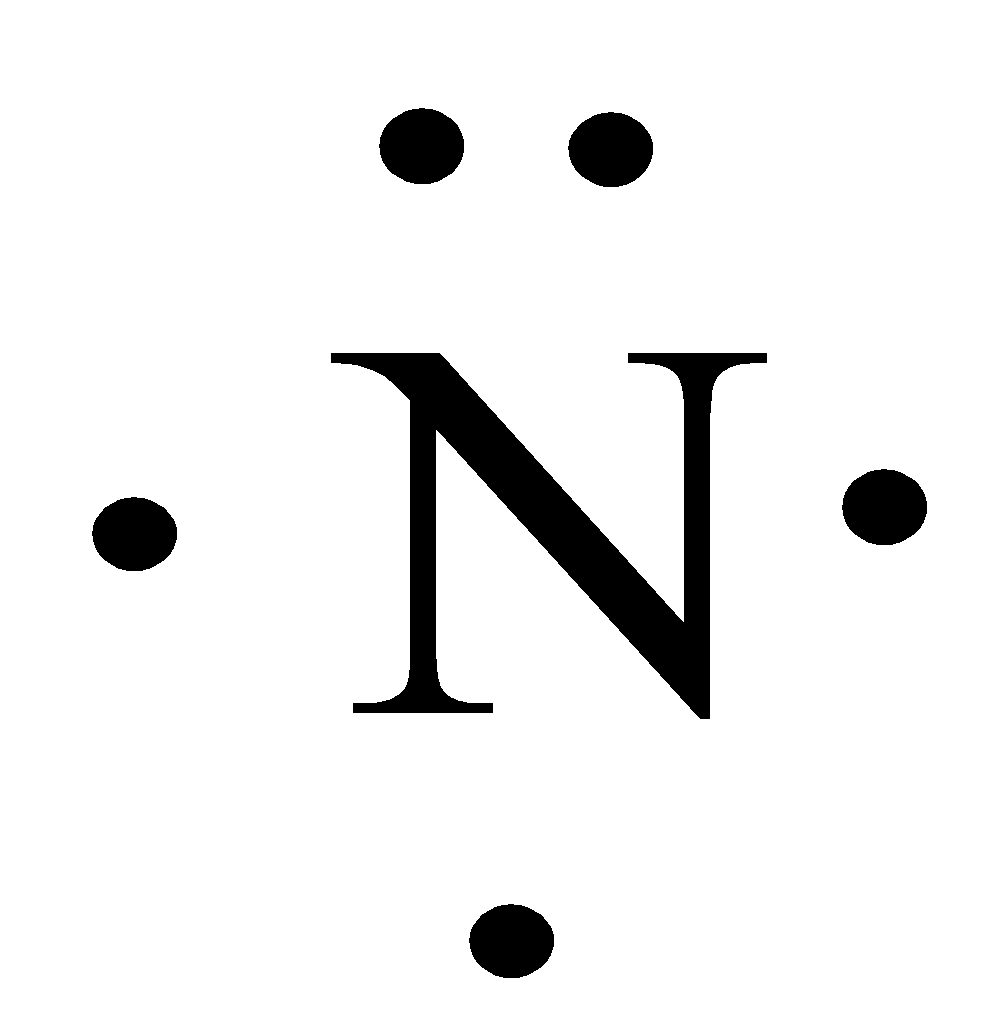
When you draw the Lewis structure for Nitrogen you'll put five "dots" or valance electrons around the element symbol (N). Click to see full answer Thereof, what are electron dot diagrams used for? There are shorthand ways to represent how atoms form covalent or ionic bonds.
NO2 (Nitrogen Dioxide) Lewis Dot Structure. Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3.
What is the Lewis dot structure for ammonia? In the lewis structure of ammonia (NH3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Can you draw the electron dot structure of […]
Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure. N 2 lewis structure There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.
Lewis dot structures (or just Lewis structures) were developed around 1920 by pioneering chemist Gilbert Lewis, as a way of picturing chemical bonding in molecules.. We draw Lewis structures to . Discover the bonding arrangement of atoms,; Discover whether there is any degeneracy of bonding (more on that later),; Figure out whether a given group of atoms might even bond together to form a ...
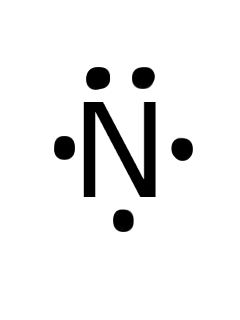
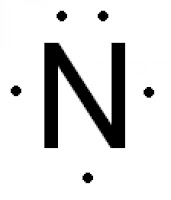
The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. .. . N . .
How is the Lewis structure for nitrogen dioxide drawn? NO2 has 5+6+6=17 valence electrons. N is in the center and sp2 hybridized to a triangular planar shape. One O atom is double bonded and has two nonbonded electron pairs on it. The other O atom is single bonded with three nonbonded electron pairs. There is a single electron on the N atom.
The nitrogen is the central atom and there is one lone pair on it. The lewis structure of NH2OH has a total of 3 lone pairs and 4 bond pairs. NH2OH lewis structure is drawn with the same procedure as the NH2Cl lewis structure. Follow some steps for drawing the lewis dot structure for NH2OH 1. Count total valence electron in NH2OH
NO2 Lewis Dot Structure. Whenever something burns in the air, Nitrogen oxides will be formed. The reason for this is that the air we breathe mainly consists of Nitrogen (78%) and Oxygen (21%), and these combine in the presence of energy (from burning materials) in the environment.
:max_bytes(150000):strip_icc()/NO2_Dot-56a12a2c3df78cf772680359.png)




![Draw the electron dot structure of Nitrogen molecule [N = 7]](https://haygot.s3.amazonaws.com/questions/1890007_1909574_ans_16e2a124f2974b5694de1a9f3c97eebd.png)
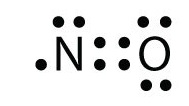






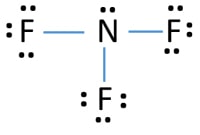










0 Response to "40 lewis dot diagram of nitrogen"
Post a Comment