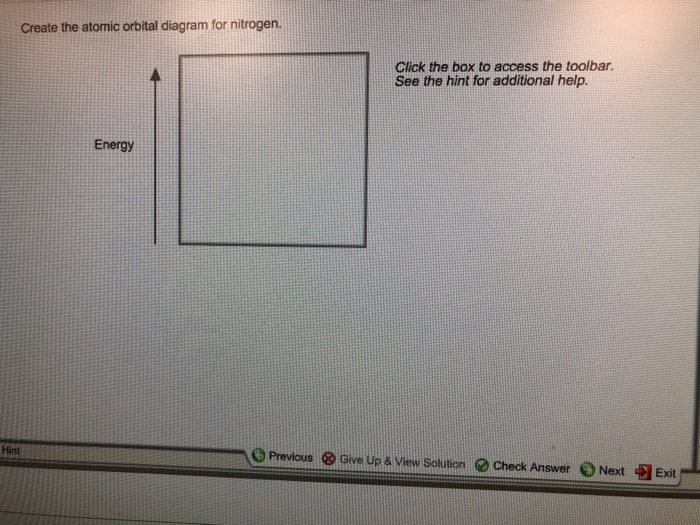
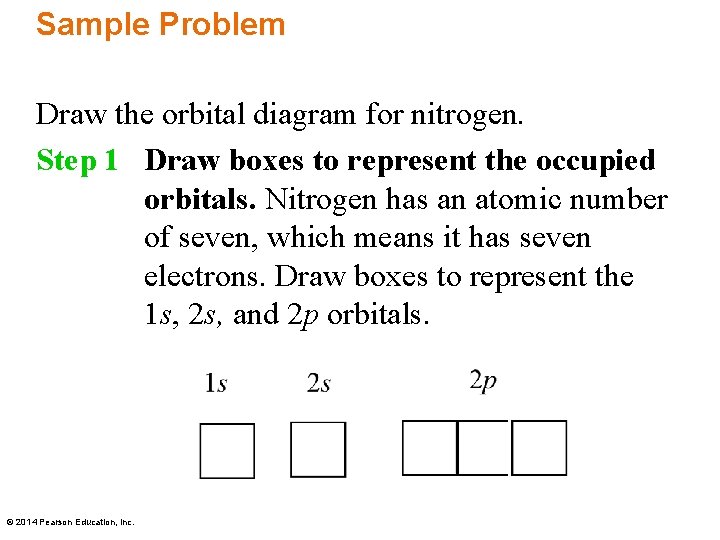
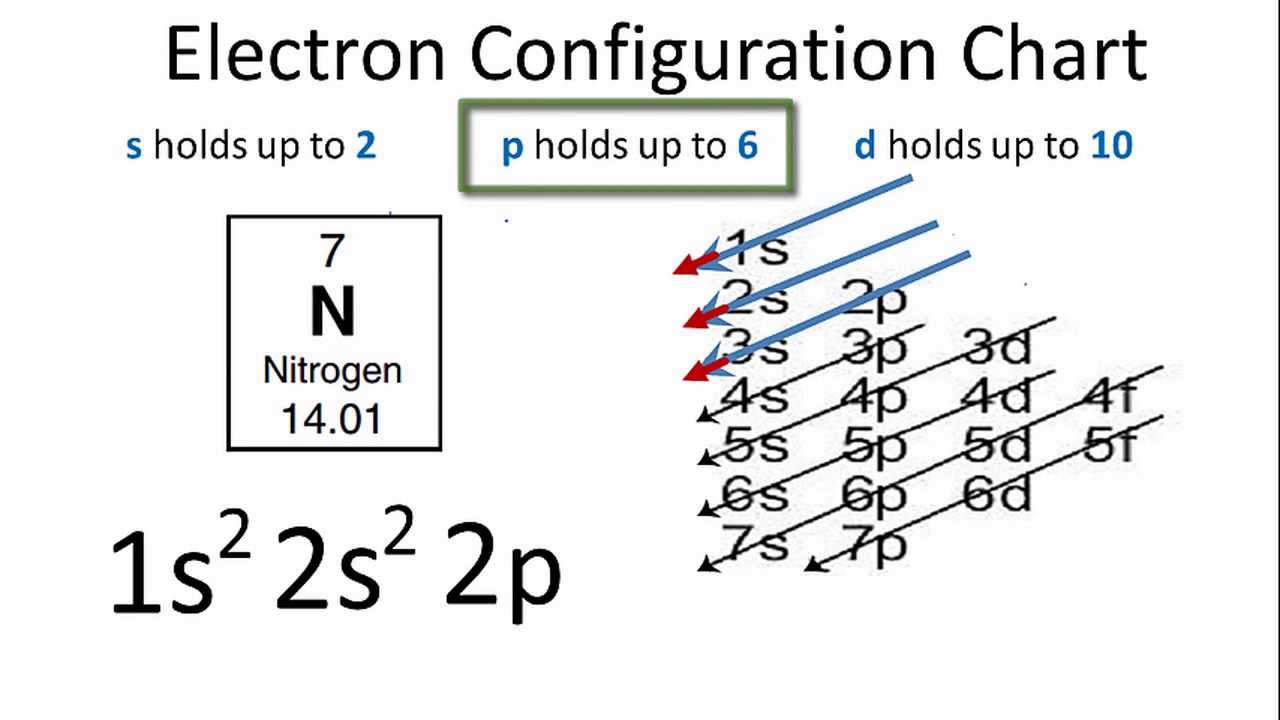
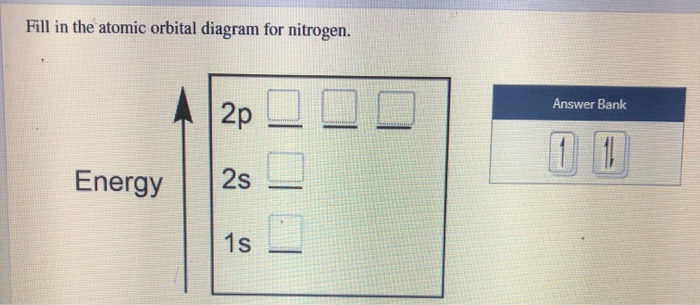
41 create the atomic orbital diagram for nitrogen.
The atomic number of Vanadium is 23 and the unique thing about the atomic number is that it is different and no two atomic numbers can be the same. For a student, it is very important to know the basics of chemistry, and knowing terms such as electronic configuration and atomic number is a must. 1540s, "of or pertaining to the eye socket;" 1839 with reference to heavenly bodies; from orbit (n.) + -al (1).
Start studying Orbital Diagrams, Electron Configuration, Valence electrons. ... The arrows represent the 16 electrons of the sulfur atom and the directions .... The Bohr model was a one-dimensional model that used one quantum number to describe the distribution of electrons in the atom.

Create the atomic orbital diagram for nitrogen.
Tagged as: #digitalkemistry, Bond order of Nitrogen molecule, chemical bonding, chemical bonding and shapes of molecules, chemistry, class 11 chemistry molecular orbital theory, class 11 chemistry molecular orbital theory notes, class 11 chemistry notes, digital kemistry, How do you draw a molecular orbital diagram, How do you find the bond ... The electrical current properties of single-molecule sensing devices based on electronic (tunneling) transport strongly depend on molecule frontier orbital energy, spatial distribution, and position with respect to the electrodes. Here, we present an analysis of the bias dependence of molecule frontier orbital properties at an exemplar case of DNA nucleotides in the gap between H-terminated (3 ... also intraorbital, 1836, from intra- "within" + orbit (n.) + -al (1).
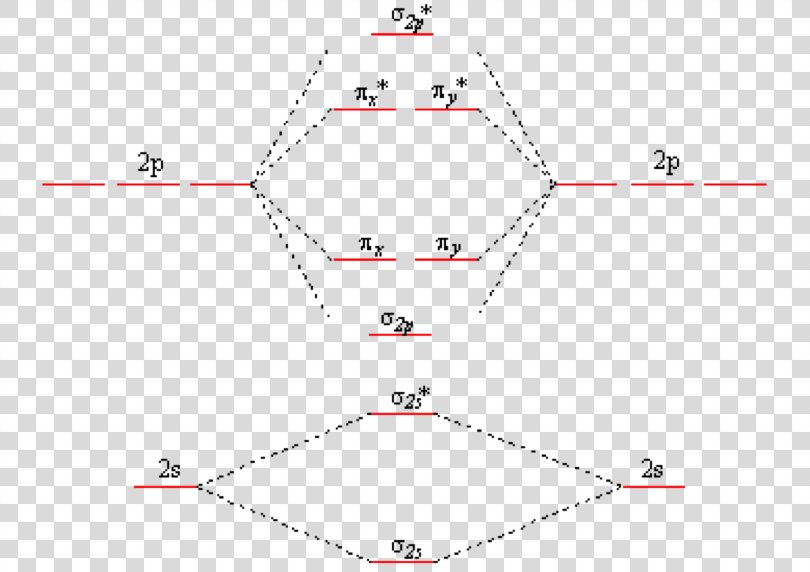
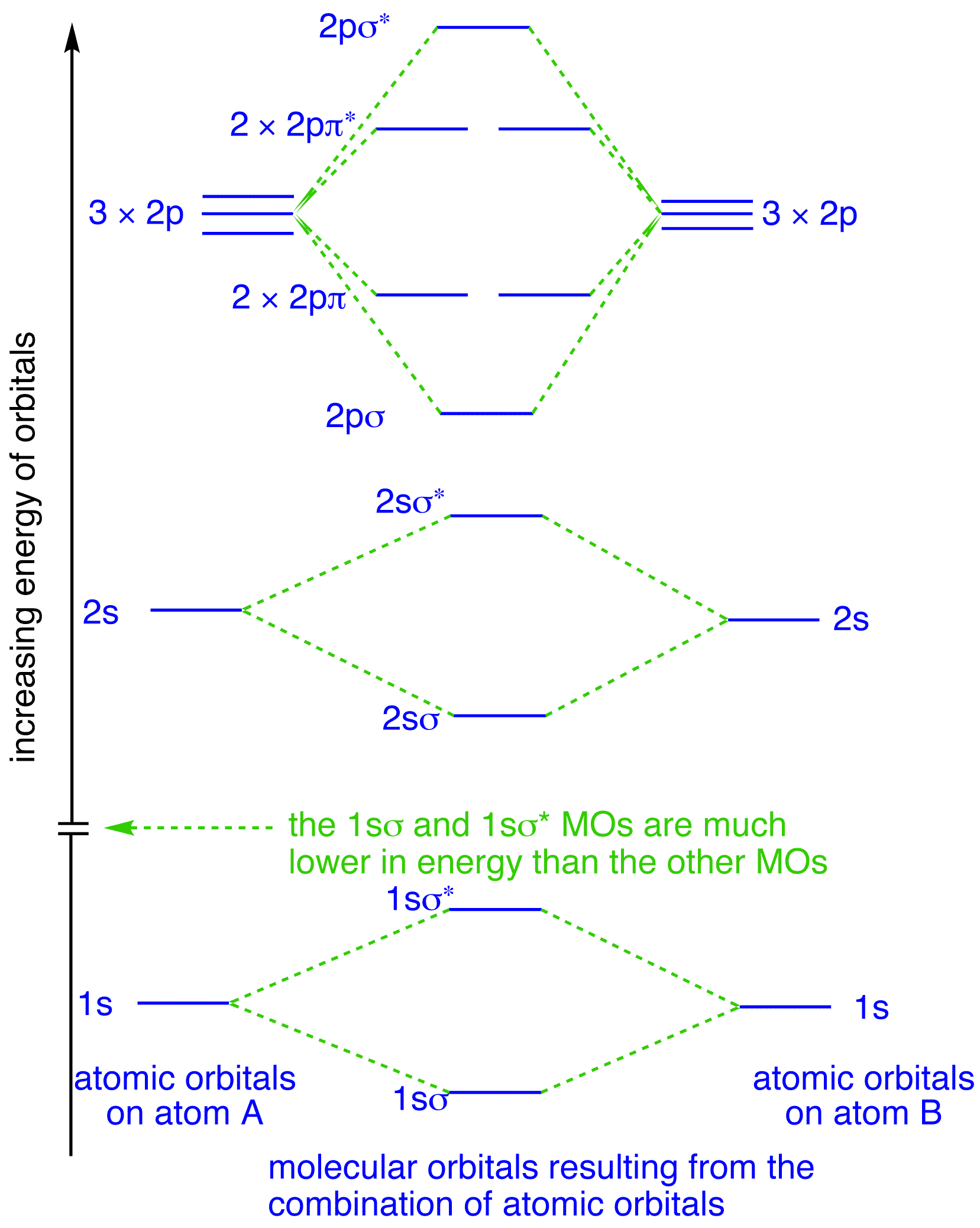
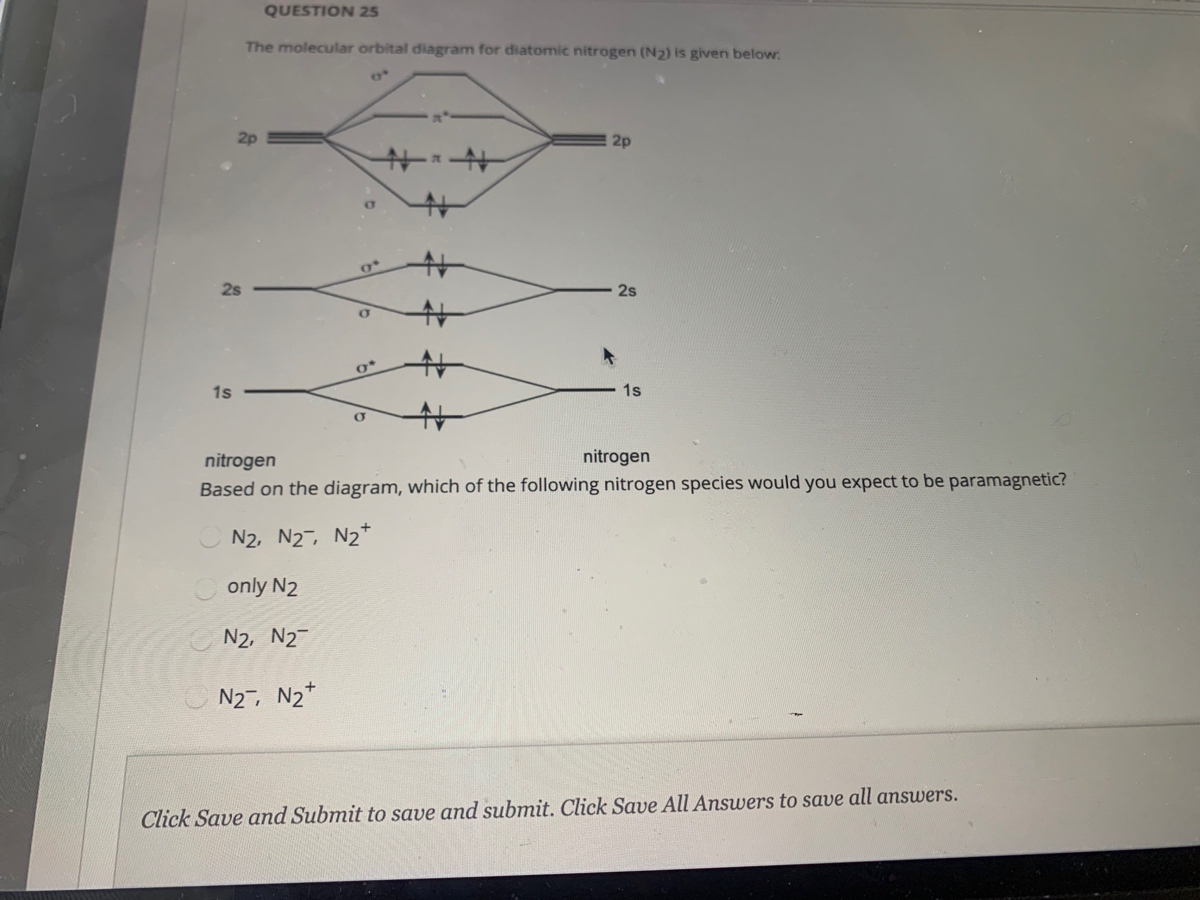
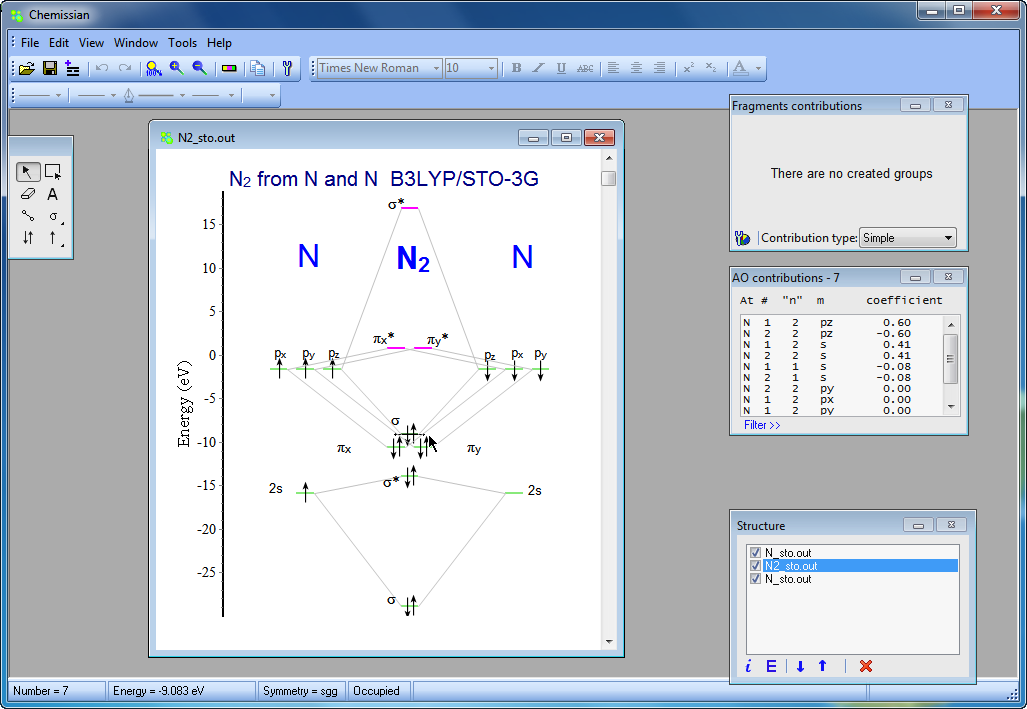
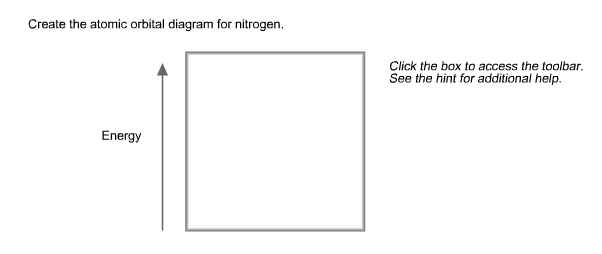
Create the atomic orbital diagram for nitrogen.. In the molecular orbital theory, two atomic orbitals of valence electrons of similar energy and orientation form a bond. Molecular orbital arises from two atomic orbitals, which have to be conserved. Figure 6.29 tells us that the next lowest power orbital is 2 s, therefore the orbital diagram for lithium is. with 3 unpaired electrons. The electron configuration of nitrogen is therefore 1 s 22 s 22 p 3. At oxygen, v Z = 8 and also eight electrons, we have actually no choice. Something like Diagram #2 below, which implies a very different degree of hybridization (none). Just to be clear, "N 3" means the nitrogen molecule (which is N 2). DIAGRAM #2. The four orbitals (2 sigma atomic and 2 pi atomic) interact and separate into 4 sigma bonds, two bonding and two antibonding. The lowest three sigma MOs are filled in N 2 ... John Deere Auger Parts Diagram for 54" two stage snowblower. Easy to use schematic for quickly buying oem augers parts. John Deere Snow Blowers & Snow Throwers parts with OEM John Deere parts diagram s to find John Deere Snow Blowers & Snow Throwers repair parts quickly and easily. Order Status Customer Support 512-288-4355 My Account.
Create the atomic orbital diagram for nitrogen. Start by adding the appropriate subshells. For example, boron is in the 2p block of the periodic table, and so ... The atomic number of Carbon is 6 so 2 electrons are filled in 's' orbital and the rest 4 are in the outer orbital that is why the valence number of electrons in carbon is 4. For Nitrogen, its atomic number is 7, so after 2 electrons occupy 's' orbital, the rest 5 are in the outer orbital so the valence number of electrons is 5. prefix usually meaning "away, opposite, completely," from Old English for-, indicating loss or destruction, but in other cases completion, and used as well with intensive or pejorative force, from Proto-Germanic *fur "before, in" (source also of Old Norse for-, Swedish för-, Dutch ver-, Old High German fir-, German ver-); from PIE *pr-, from root *per- (1) "forward," hence "in front of, before, toward, near, against." In verbs the prefix denotes (a) intensive or completive action or process, or (b) action that miscarries, turns out for the worse, results in failure, or produces adverse or opposite results. In many verbs the prefix exhibits both meanings, and the verbs frequently have secondary and figurative meanings or are synonymous with the simplex. [Middle English Compendium] Probably originally in Germanic with a sense of "forward, forth," but it spun out complex sense developments in the historical languages. Disused as a word-forming element in Modern English. Ultimately from the same root as fore (adv The above image shows the lewis Structure of single nitrogen and a hydrogen atom. The atomic number of the nitrogen is seven, which makes its electronic configuration 1s2 2s2 2p3. As the p shell needs to accommodate a maximum of six electrons, there is a scarcity of three electrons. It makes a single nitrogen atom to have five valence electrons.
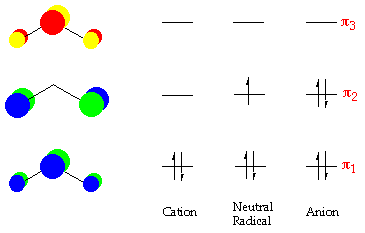
Nitrogen is the chemical element with the symbol N and atomic number 7. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772. Although Carl Wilhelm Scheele and Henry Cavendish had independently done so at about the same time, Rutherford is generally accorded the credit because his work was published first. The name nitrogène was suggested by French chemist Jean ... colorless, odorless gaseous element, 1794, from French nitrogène, coined 1790 by French chemist Jean Antoine Chaptal (1756-1832), from Greek nitron "sodium carbonate" (see nitro-) + French gène "producing," from Greek -gen "giving birth to" (see -gen). The gas was identified in part by analysis of nitre. An earlier name for it was mephitic air (1772), and Lavoisier called it azote (see azo-). It forms about 78% of the weight of the Earth's atmosphere. Related: Nitrogenic; nitrogenous. Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two ... So here, we need to create an atomic orbital diagram for nitrogen. Let's go ahead and write its electron configuration first, though, ...Apr 5, 2018
Why do you think these elements are grouped in. The electron configuration and the orbital diagram are: Lithium has 3 protons, compared to 11 in sodium. After clicking check, note the electron configuration and the atomic radius now listed at right. Compare the electron configurations of hydrogen, lithium, and sodium.
Create the atomic orbital diagram for nitrogen; Shark drags woman into crocodile; Political corruption during reconstruction was; A fish that has a flexible skeleton made of cartilage is known as a; Purple and gold backgrounds; Used smith and wesson m&p 15-22 for sale; Out and about burger
Residential solar swimming pool heater s are usually plumbed using the same size pvc pipe as the pool equipment, normally 2" or 1.5". Notice in the typical arrangement shown in this schematic that filtered water flows through solar and then through the gas heater if there is one. Independent system. Regardless of the heating type - solar, gas, heat pump or a combination, we recommend where ...
Create the atomic orbital diagram for nitrogen. Start by adding the appropriate subshells. For example, boron is in the 2p block of the periodic table, and so you need to show the 2p subshell and everything below it. Next, click the orbitals to add...
Which nitrogen atom(s) is/are sp 3 hybridized. 5. Describe the bonding scheme of CH 4. Answers: 1. a and b. 2. Just like the energy diagram in fig.3. For carbon, each sp 3 orbital has 1 electron. For nitrogen, the first sp 3 orbital has 2 electrons, then one electron for each of the remaining three. 3. All of them (Don't for get the elctron ...
Walsh Diagram For Tri And Penta Atomic Molecules Pdf 98 explain walsh diagram for tri and penta atomic molecules, draw the walsh diagram .... 1.4.2 Application to penta atomic molecules. 1.5 Dp- pp ... Figure 1.8 Orbital Correlation Diagram For AB2 Triatomic Molecules Where A ... 98 californium (Cf)..
This orbital diagram for nitrogen shows how electrons fill the sub-orbitals. This is the electron configuration for the element sulfur, which has 16 electrons. We and everything around us are made ...
Jan 21, 2021 — When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next ...
Atomic nitrogen has 5 valence electrons and 4 valence orbitals (2s, 2px, 2py, and 2pz). In the Lewis structure there is a triple bond between the nitrogen ...
1918 (Venn's diagram is from 1904), named for English logician John Venn (1834-1923) of Cambridge, who explained them in the book "Symbolic Logic" (1881).
Entities are nouns. Create the diagram and entities. In Visio, on the File menu, select New > Software, and then select Crow's Foot Database Notation.. Choose either Metric Units or US Units, and select Create. From the Crow's Foot Database Notation stencil, drag an Entity shape onto the drawing page.. Drag another Entity shape onto the ...
Step 3: Construct the orbital diagram for the ion. 82% (439 ratings) Procedure for Construct ing Molecular Orbital Diagram s B as ed on Hybrid Orbital s 1. Begin with the Lewis structure. 2. Decide how many orbital s each atom needs to make its sigma bonds and to hold its non-bonding electrons.
Electronic configuration of C l, Atomic number = 1 7, E. C of C l = 1 s 2 2 s 2 2 p 6 3 s 2 3 p 5. Chlorine Electron Configuration - YouTube Study Guide Chemistry Archive | September 18, 2016 | Chegg.com The Aufbau Principle | Study.com Electron Configuration Solved: Create The Atomic Orbital Diagram For Nitrogen.
FREE Answer to 1. Create the atomic orbital of diagram for nitrogen. 2. Construct the orbital diagram for Ni.1 answer · 0 votes: Nitrogen rightarrow 7 rightarrow 1 s^2 2 s^2 2p^3 orbital diagram (2) Ni rightarrow 28 rightarrow 1 s^2 2s^2 2p^6 3s^2 3p^6 3d^8 4s^2 orbital diagram:
"to bring into being," early 15c., from Latin creatus, past participle of creare "to make, bring forth, produce, procreate, beget, cause," related to Ceres and to crescere "arise, be born, increase, grow," from PIE root *ker- (2) "to grow." De Vaan writes that the original meaning of creare "was 'to make grow', which can still be found in older texts ...." Related: Created; creating.
In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...Oct 24, 2016 · Uploaded by Wayne Breslyn
1610s, "an illustrative figure giving only the outlines or general scheme of the object;" 1640s in geometry, "a drawing for the purpose of demonstrating the properties of a figure;" from French diagramme, from Latin diagramma "a scale, a musical scale," from Greek diagramma "geometric figure, that which is marked out by lines," from diagraphein "mark out by lines, delineate," from dia "across, through" (see dia-) + graphein "write, mark, draw" (see -graphy). Related: Diagrammatic; diagrammatically. The verb, "to draw or put in the form of a diagram," is by 1822, from the noun. Related: Diagrammed; diagramming.
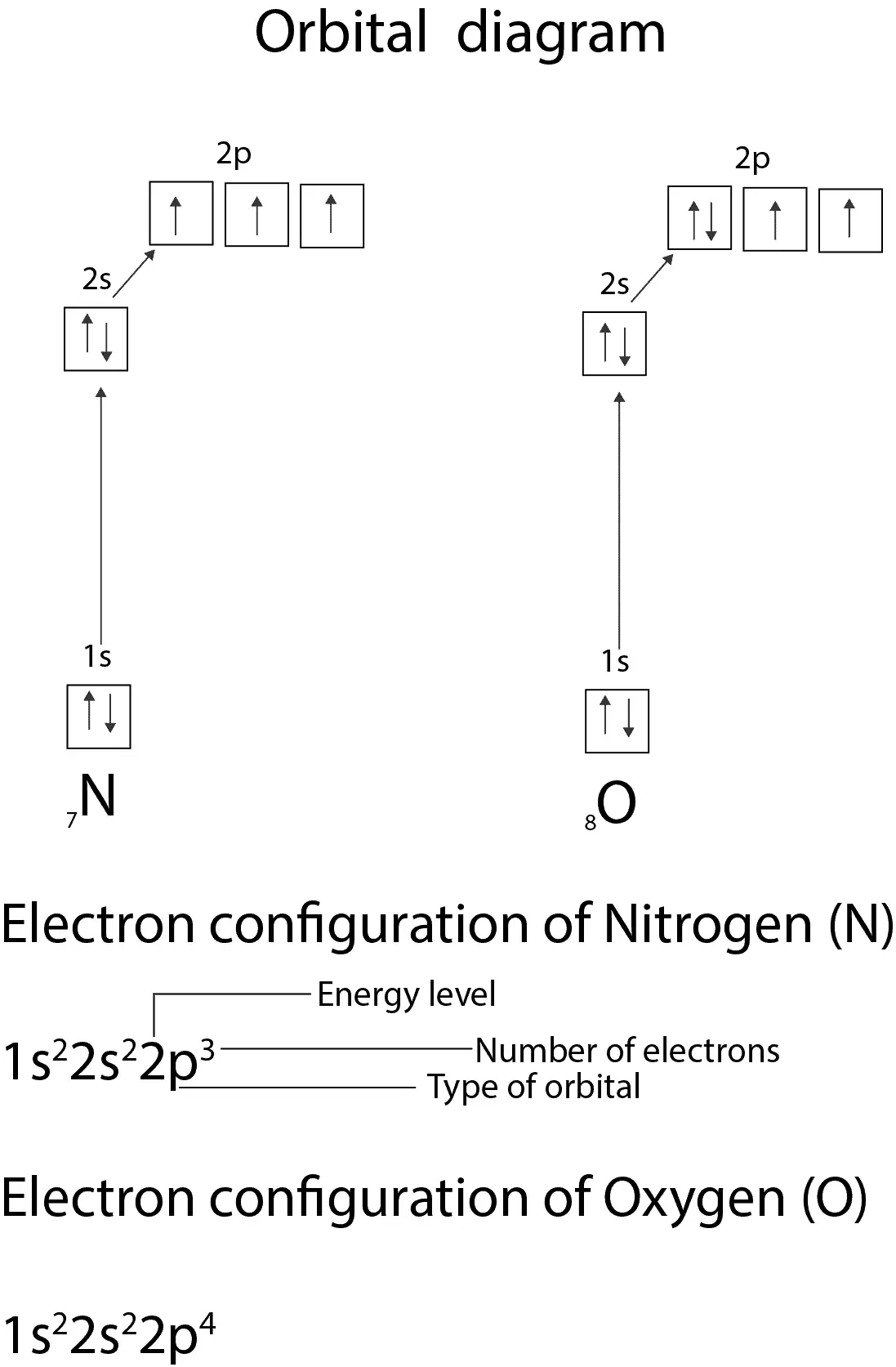
What is the atomic orbital diagram for nitrogen? The remaining three electrons will go in the 2p orbital. Therefore the N electron configuration will be 1s22s22p3. The configuration notation for Nitrogen (N) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the Nitrogen atom.
Atomic no. Orbital Diagram of All Elements Diagrams; 1: Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram ...
(d) Nitrogen and Oxygen. These atoms each have seven and eight electrons. After six electrons have been accommodated in the manner described above, a vacant \(2{{\text{p}}_{\text{z}}}\) orbital remains, which will be the seat of the seventh electron with the same spin orientation.
The bonding molecular orbital is spread over the nitrogen and both oxygen atoms. C Placing 4 electrons in the energy-level diagram fills both the bonding and nonbonding molecular orbitals and gives a π bond order of 1/2 per N-O bond. The overall N-O bond order is 1 1 2, consistent with a resonance structure.
Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p.Show transcribed image text Show the orbital-filling diagram for N. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital.
1 answerWe are told to create the atomic orbital diagram of Nitrogen. For this we will write the electronic configuration of Nitrogen atom which has 7...
40 strip diagram anchor chart; 37 orbital diagram for barium; 37 4 string day night shade diagram; 42 create the atomic orbital diagram for nitrogen. 39 1955 chevy ignition switch wiring diagram; 41 john deere 325 parts diagram; 41 choose the correct orbital diagram for vanadium. 38 chevy s10 stereo wiring diagram; 42 john deere d110 belt diagram
1550s, "be taken or regarded as," also "be in favor of," from go (v.) + for (adv.). Meaning "attack, assail" is from 1880. Go for broke is from 1951, American English colloquial.
Electron Configuration For Nitrogen Ion. The atomic number of nitrogen is 7, the element nitrogen was discovered by a Scottish physician, Danial Rutherford. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element Nitrogen.
Old English for "before, in the sight of, in the presence of; as far as; during, before; on account of, for the sake of; in place of, instead of," from Proto-Germanic *fur "before; in" (source also of Old Saxon furi "before," Old Frisian for, Middle Dutch vore, Dutch voor "for, before;" German für "for;" Danish for "for," før "before;" Gothic faur "for," faura "before"), from PIE root *per- (1) "forward," hence "in front of, before," etc. From late Old English as "in favor of." For and fore differentiated gradually in Middle English. For alone as a conjunction, "because, since, for the reason that; in order that" is from late Old English, probably a shortening of common Old English phrases such as for þon þy "therefore," literally "for the (reason) that."
"pertaining to atoms," 1670s as a philosophical term (see atomistic); scientific sense dates from 1811, from atom + -ic. Atomic number is from 1821; atomic mass is from 1848. Atomic energy first recorded 1906 in modern sense (as intra-atomic energy from 1903). March, 1903, was an historic date for chemistry. It is, also, as we shall show, a date to which, in all probability, the men of the future will often refer as the veritable beginning of the larger powers and energies that they will control. It was in March, 1903, that Curie and Laborde announced the heat-emitting power of radium. [Robert Kennedy Duncan, "The New Knowledge," 1906] Atomic bomb first recorded 1914 in writings of H.G. Wells ("The World Set Free"), who thought of it as a bomb "that would continue to explode indefinitely." When you can drop just one atomic bomb and wipe out Paris or Berlin, war will have become monstrous and impossible. [S. Strunsky, Yale Review, January 1917] Atomic Age is from 1945. Atomical "concerned with atoms," also "ve
also intraorbital, 1836, from intra- "within" + orbit (n.) + -al (1).
The electrical current properties of single-molecule sensing devices based on electronic (tunneling) transport strongly depend on molecule frontier orbital energy, spatial distribution, and position with respect to the electrodes. Here, we present an analysis of the bias dependence of molecule frontier orbital properties at an exemplar case of DNA nucleotides in the gap between H-terminated (3 ...
Tagged as: #digitalkemistry, Bond order of Nitrogen molecule, chemical bonding, chemical bonding and shapes of molecules, chemistry, class 11 chemistry molecular orbital theory, class 11 chemistry molecular orbital theory notes, class 11 chemistry notes, digital kemistry, How do you draw a molecular orbital diagram, How do you find the bond ...























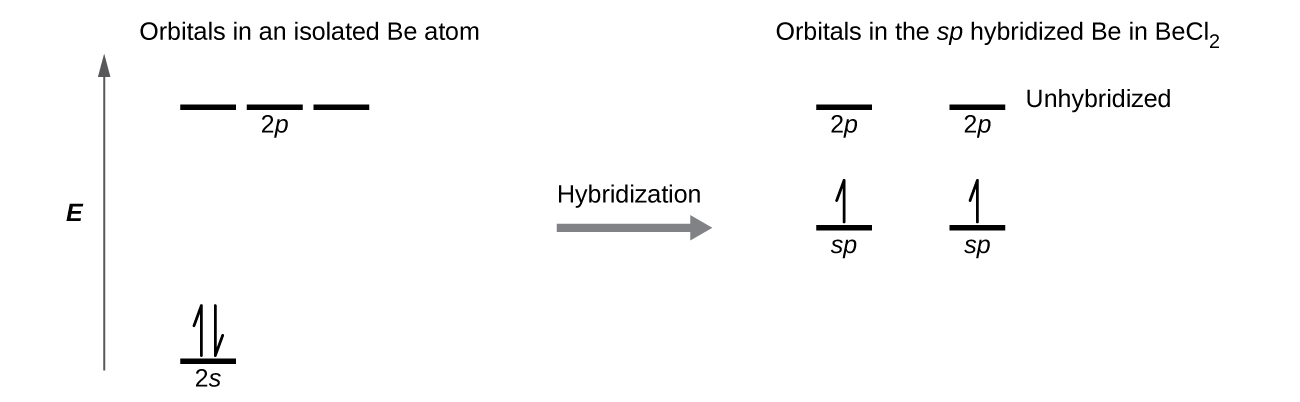
0 Response to "41 create the atomic orbital diagram for nitrogen."
Post a Comment