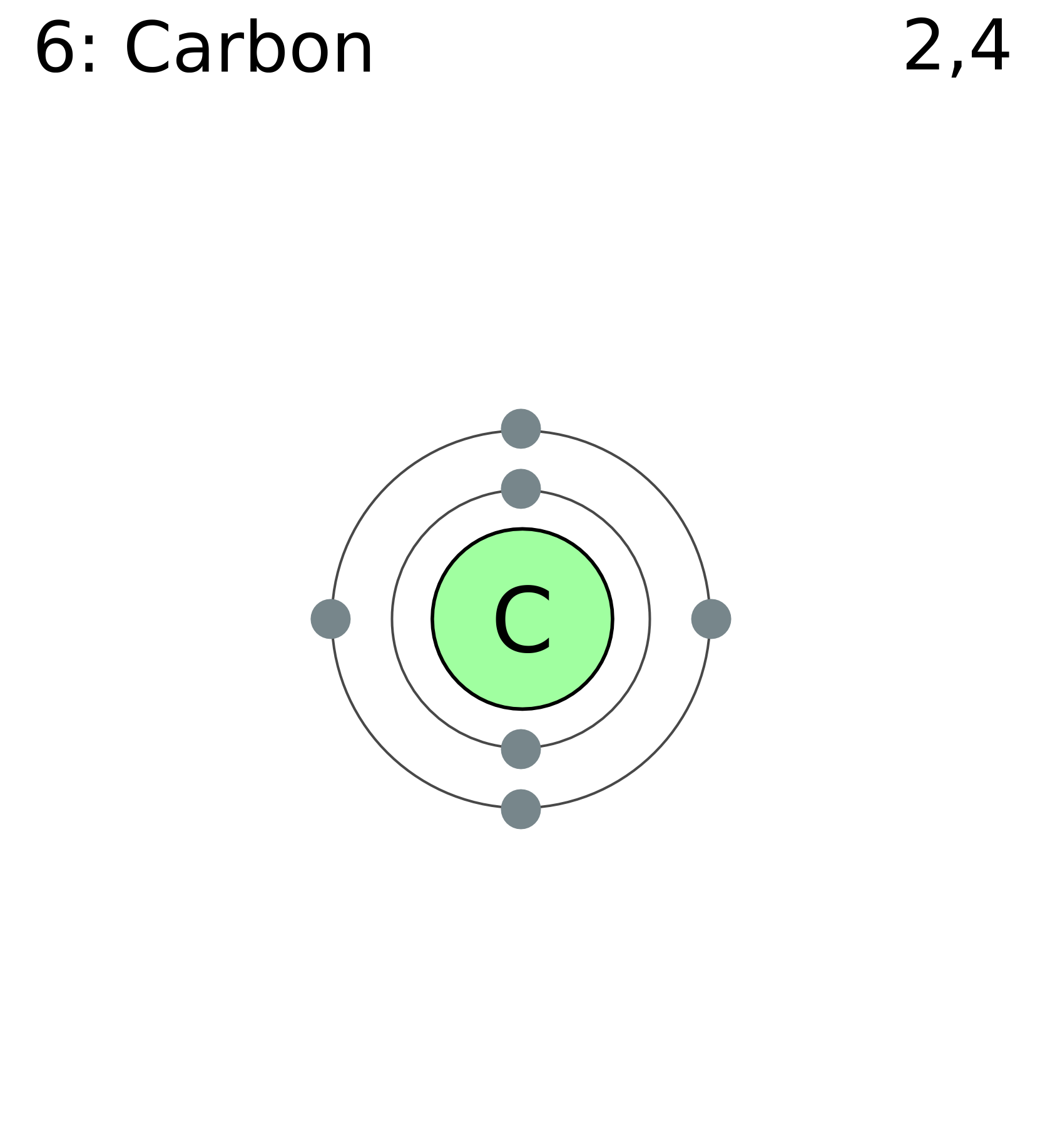
45 electron distribution diagram of carbon
Carbon nanomaterials such as carbon nanotubes (CNTs) and graphene have an extraordinary combination of mechanical, electronic and thermal properties that make them extremely attractive as nanoscale reinforcements in high-performance composites. In principle, the properties of the composite material can be multifunctional and can be precisely tailored by controlling the dispersion of the ... The distribution of electrons in the sodium atom is as follows: In the first orbit or K-shell = 2 electrons. In the second orbit or L-shell = 8 electrons. In the third orbit or M-shell = 1 electron. Or, we can write the distribution of electrons in a sodium atom as 2, 8, 1. 1,56,204.
If electrons behave as particles (rigid spheres), the distribution of electrons will vary continuously as a function of angle as in Figure 1. This distribution will vary only slightly with changes in electron energy. electron beam carbon target I(θ) θ Figure 1. Particle Model Diffraction. Continuous distribution of electrons as a function of ...

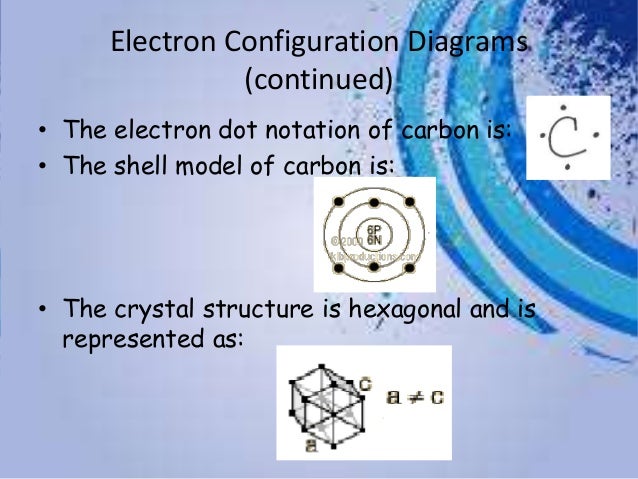
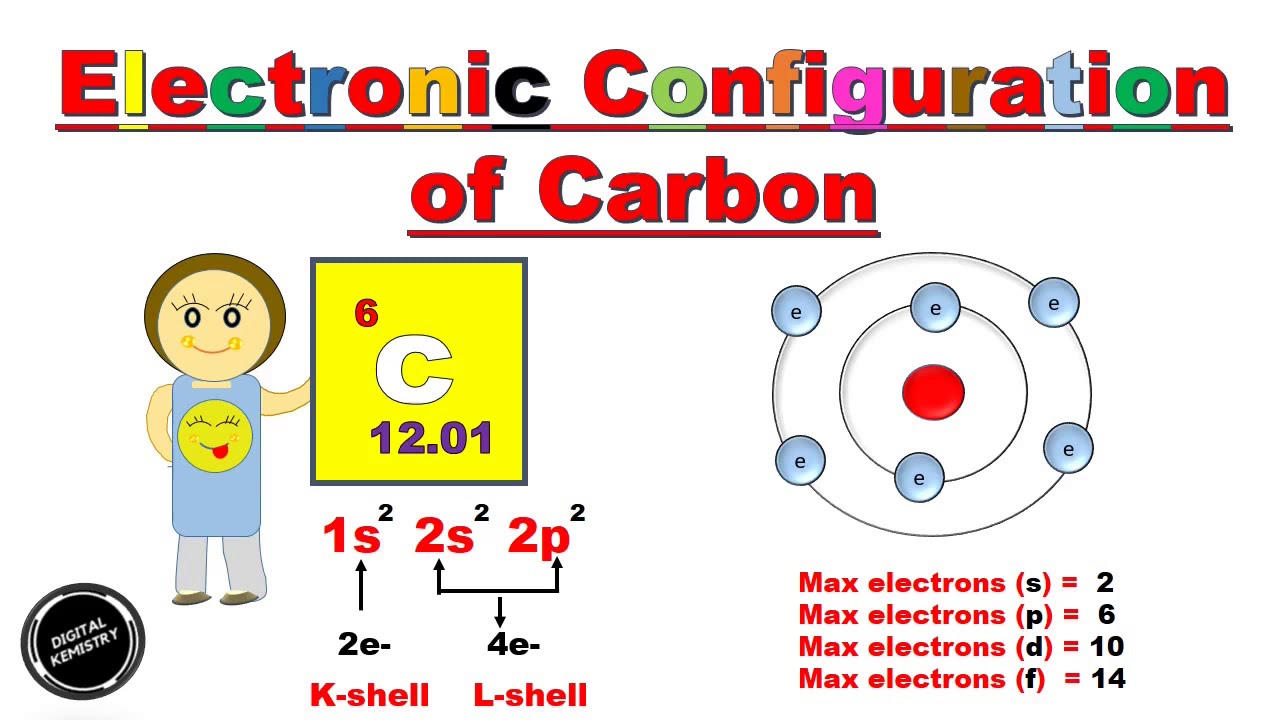
Electron distribution diagram of carbon
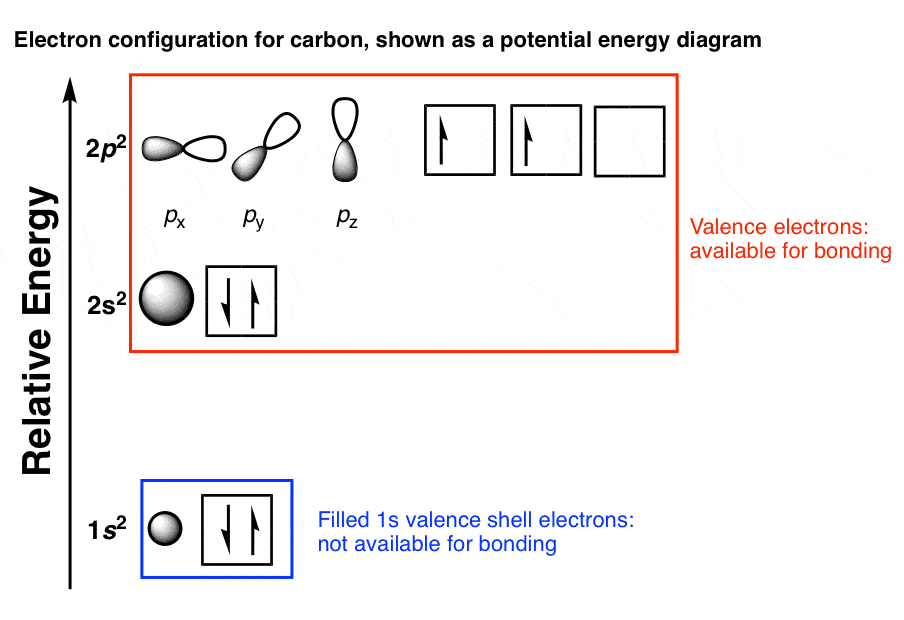
The spatial distribution of electrons occupying each of these orbitals is shown in the diagram below. Very nice displays of orbitals may be found at the following sites: J. Gutow, Univ. Wisconsin Oshkosh R. Spinney, Ohio State M. Winter, Sheffield University. The valence shell electron configuration of carbon is 2 s 2, 2p x 1, 2p y 1 & 2p z 0 ... The electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements contains all the elements in increasing order of atomic number.. To save room, the configurations are in noble gas shorthand.This means part of the electron configuration has been replaced with the element symbol of the noble gas symbol. Transcribed image text: Some Scientists think that life elsewhere in the universe might be based on the element silicon, rather than on carbon, as on Earth. Look at the electron distribution diagram for silicon, and draw the Lewis dot structure for silicon on your note. Analyze your knowledge about it and propose a short essay about the possible silicon-based life.
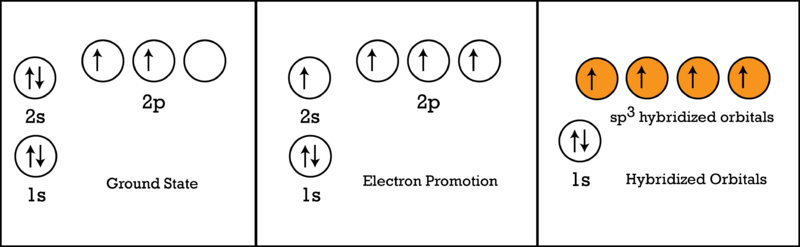
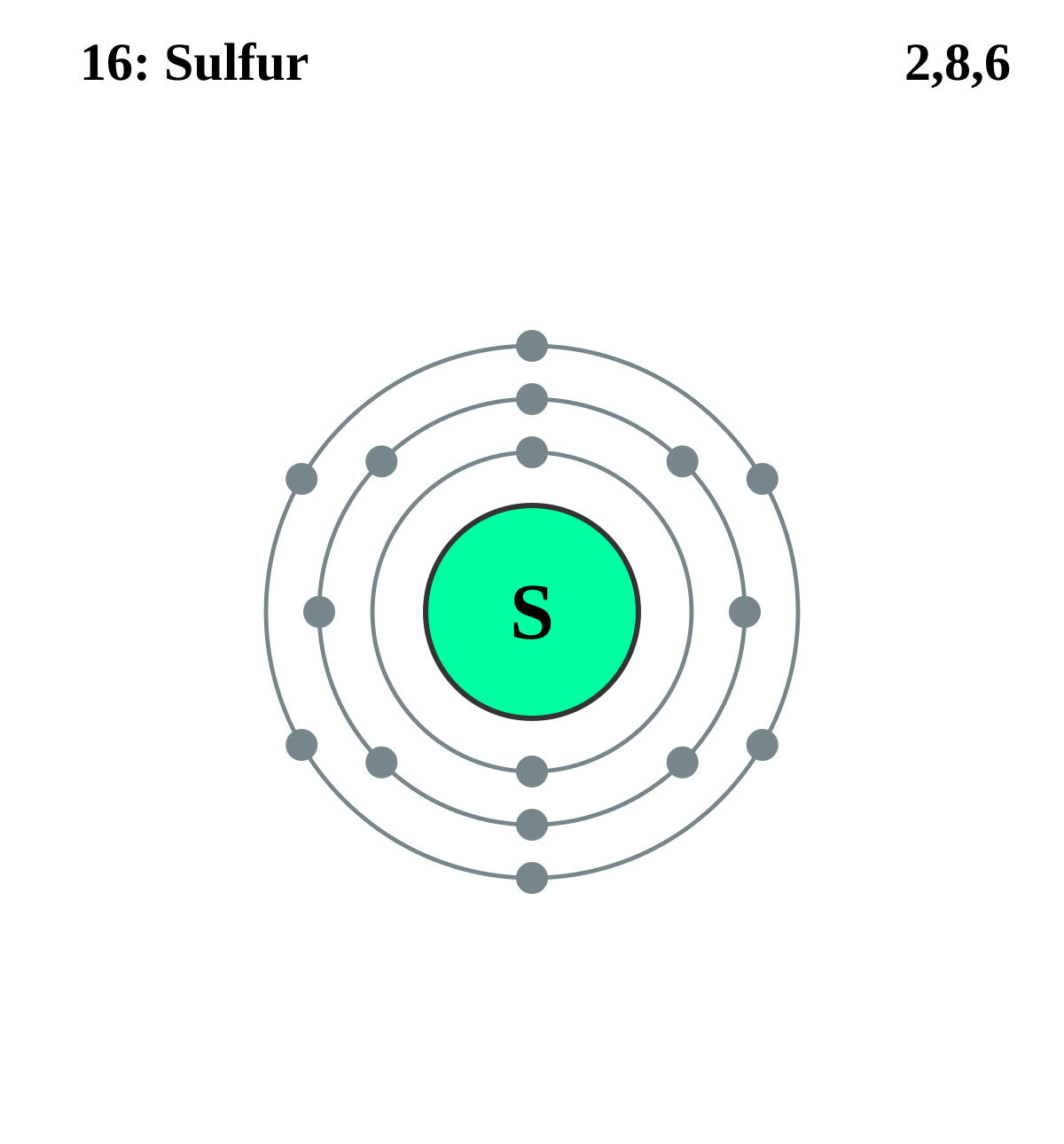
Electron distribution diagram of carbon. Make an electron distribution diagram of carbon. It is essential that you know the answers to these questions: How many valence electrons does carbon have? 4. How many bonds can carbon form? 4. What type of bonds does it form with other elements? Covalent bonds. Carbon chaines form skeletons. List the ypes of skeletons that can be formed. Carbon 6C Silicon 14Si Nitrogen 7N Phosphorus 15P Oxygen 8O Sulfur 16S Fluorine 9F Chlorine 17Cl Neon 10Ne Argon 18Ar Helium 2He 2 He Mass number 4.00 Atomic number Element symbol Electron distribution diagram Fig. 2.9: Electrons are distributed in shells of orbitals. Each orbital contains a maximum of two electrons. The electron dot diagram of an element or a molecule is called Lewis structure; it features the distribution of valence electrons around elements.. Carbon has four valence electrons and therefore, they are drawn on the four sides of a carbon atom as represented in the figures below. Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
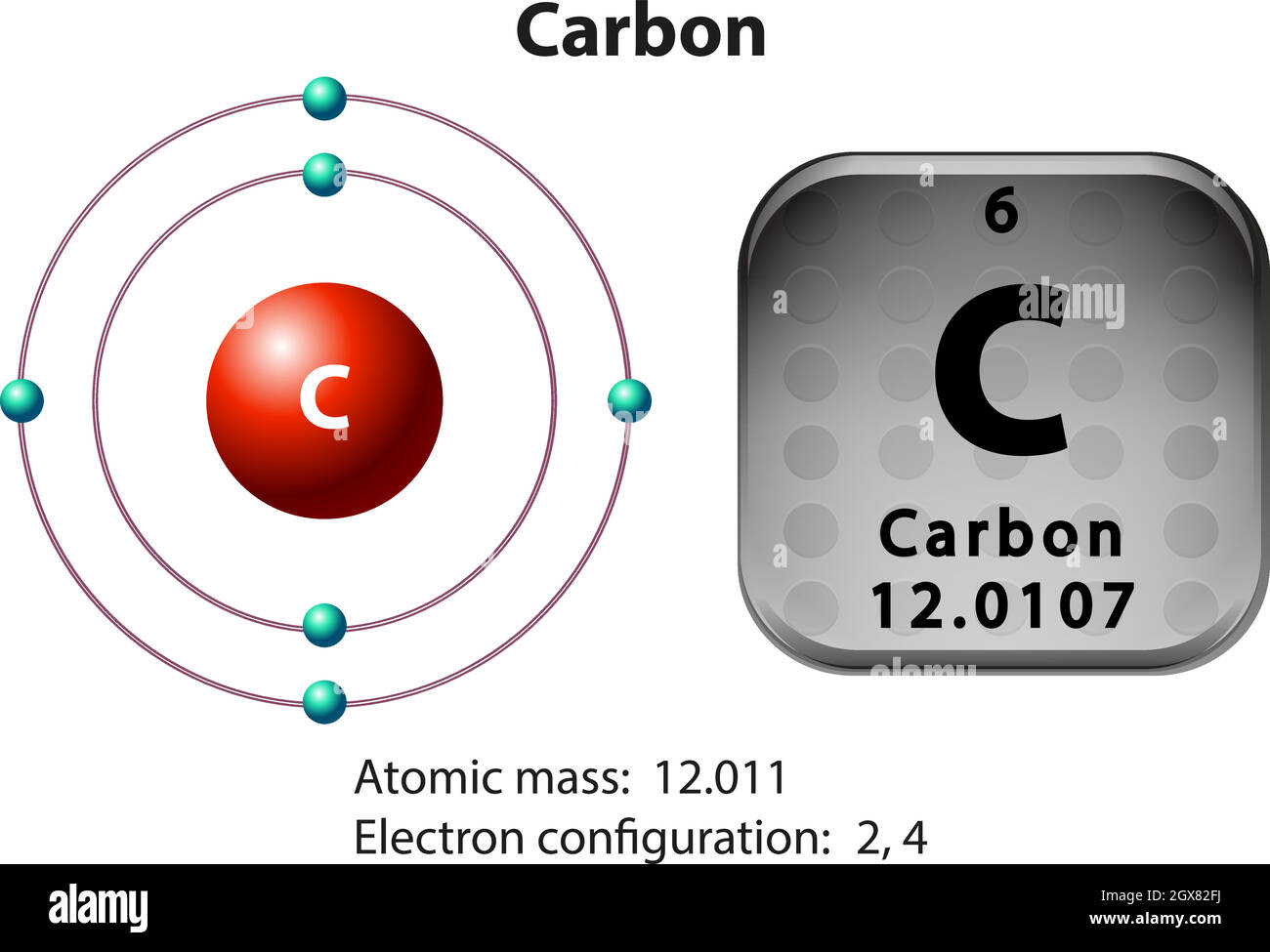
Concept 4.2 Carbon atoms can form diverse molecules by bonding to four other atoms . 3. Make an electron distribution diagram of carbon. It is essential that you know the answers to these questions: a. How many valence electrons does carbon have? b. How many bonds can carbon form? b. What type of bonds does it form with other elements? 4. Draw an electron-distribution diagram of a carbon atom Show the correct numbers, and approximate locations, of all of this atom's subatomic particles. 2a. Use the following information for your drawing: Atomic number = 6 Mass number - 12 . Verified by Toppr. CARBON : Atomic Number of Carbon = 12. Electronic Distribution : 2 , 8 , 2. Electrons in K - Shell = 2. Electrons in L - Shell = 8. Electrons in M - Shell = 2. SODIUM : Atomic Number of Sodium = 11. Electron distribution diagram of carbon luxury c6h6 benzene lewis. The remaining two electrons will go in the 2p orbital. An ion of an atom is one in which the number of protons and electrons is not the same. Make an electron distribution diagram of carbon carbon has 4 valence electrons can bond to 4 items and typically forms covalent bonds ...
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ... the distribution of electrons in the atom's electron shells Know how to find the number of valence electrons and protons of an atom from an electron distribution diagram. question 12 3. Make an electron distribution diagram of carbon. ! Carbon has 4 valence electrons, can bond to 4 items, and typically forms covalent bonds with other elements. 4. The radical, which has 1 electron in the NBMO is, as a hydrocarbon radical would be expected to be, nonpolar, i.e., it has zero charge on every atom. So, if one adds one electron to form theanion, it is added to the NBMO and the results is that the negative charge is determined by the distribution of this electron in the NBMO.
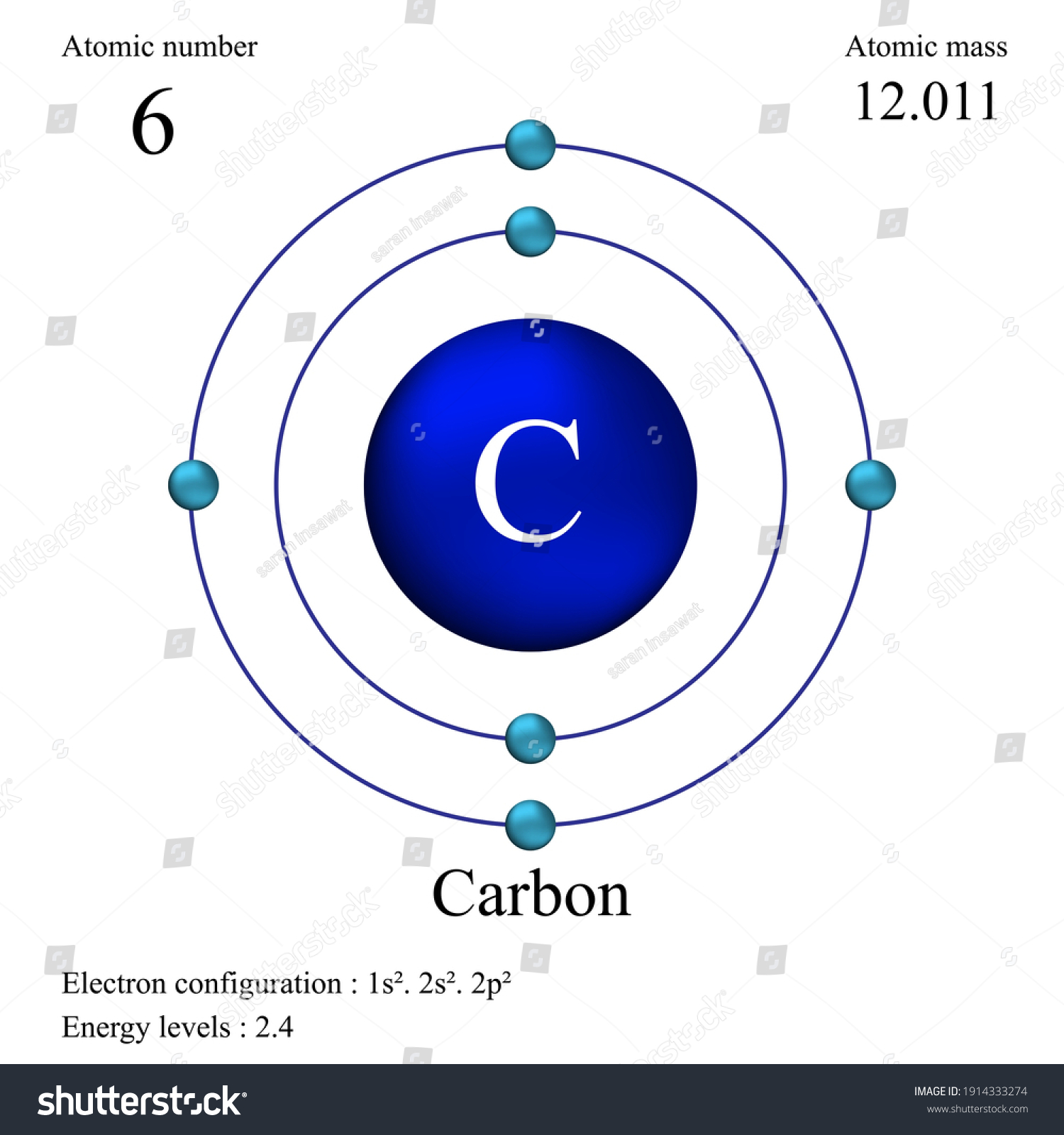
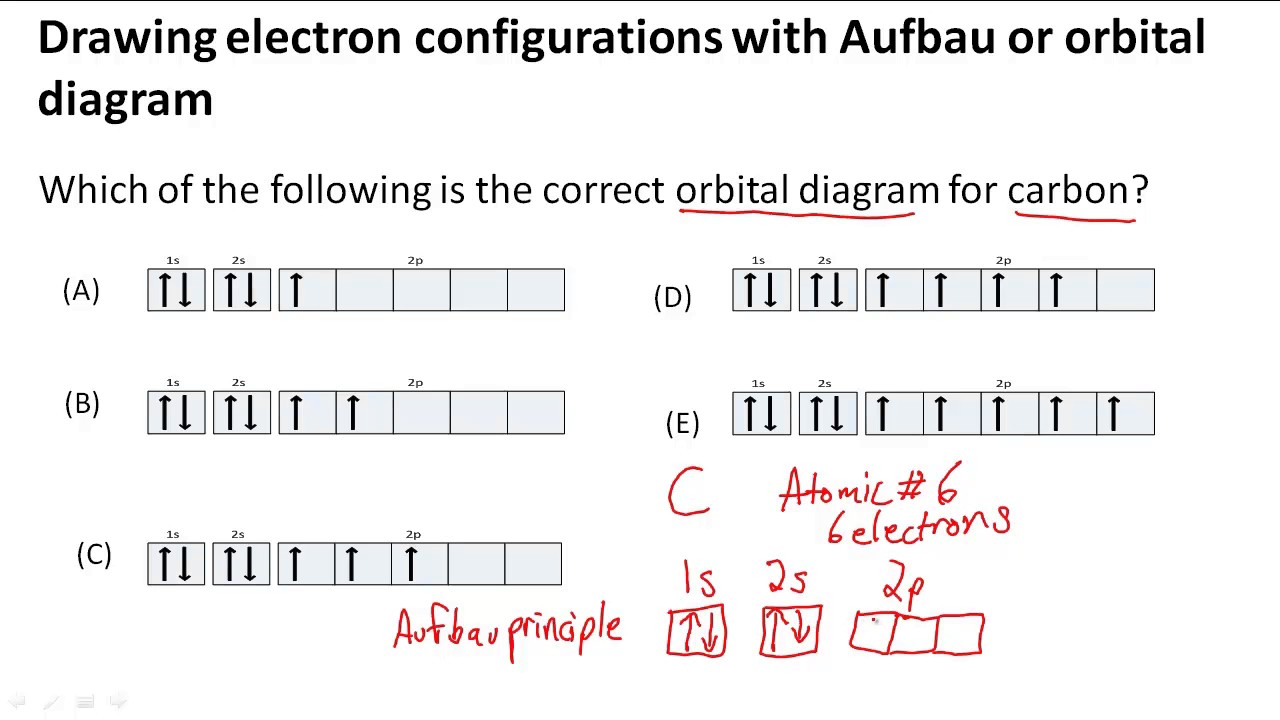
The electron configuration can be written as 1s 2 2s 2 2p 4. The orbital diagram is drawn as follows: the first 2 electrons will pair up in the 1s orbital; the next 2 electrons will pair up in the 2s orbital. That leaves 4 electrons, which must be placed in the 2p orbitals.
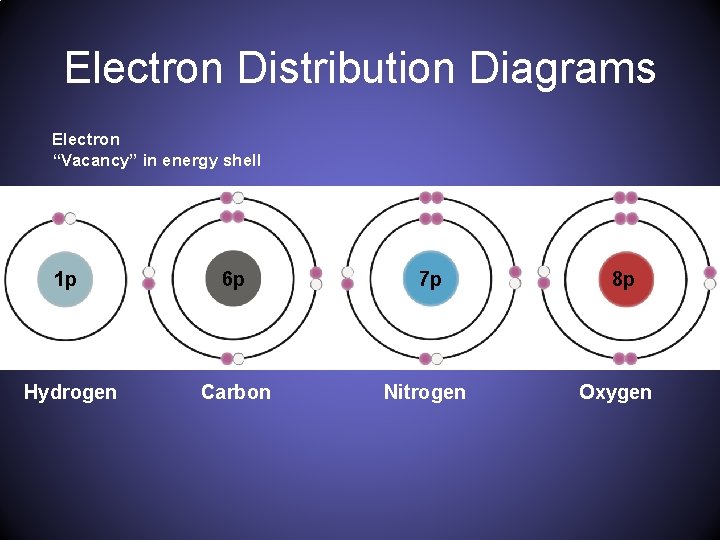
The figure below represents an electron distribution diagram where the concentric circles represent electron shells and each small circle represents an empty spot for an electron. Fill out the electron distribution diagram for the element carbon by coloring the electrons that are present; then answer the questions.
The second diagram corrects this by realizing there are two unused p orbitals on the carbon. The valence electron configuration of "O" is ["He"] 2s^2 2p^4. To accommodate the two lone pairs and the bonding pair, it will also form three equivalent sp^2 hybrid orbitals.

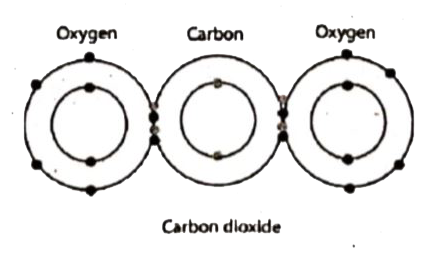
Draw The Electron Distribution Diagram For The Formation Of Carbon Dioxide Oxide Co2 Molecule Brainly In
Carbon is the sixth element with a total of 6 electrons. In writing the electron configuration for carbon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for C goes in the 2s orbital. The remaining two electrons will go in the 2p orbital. Therefore the C electron configuration will be ...
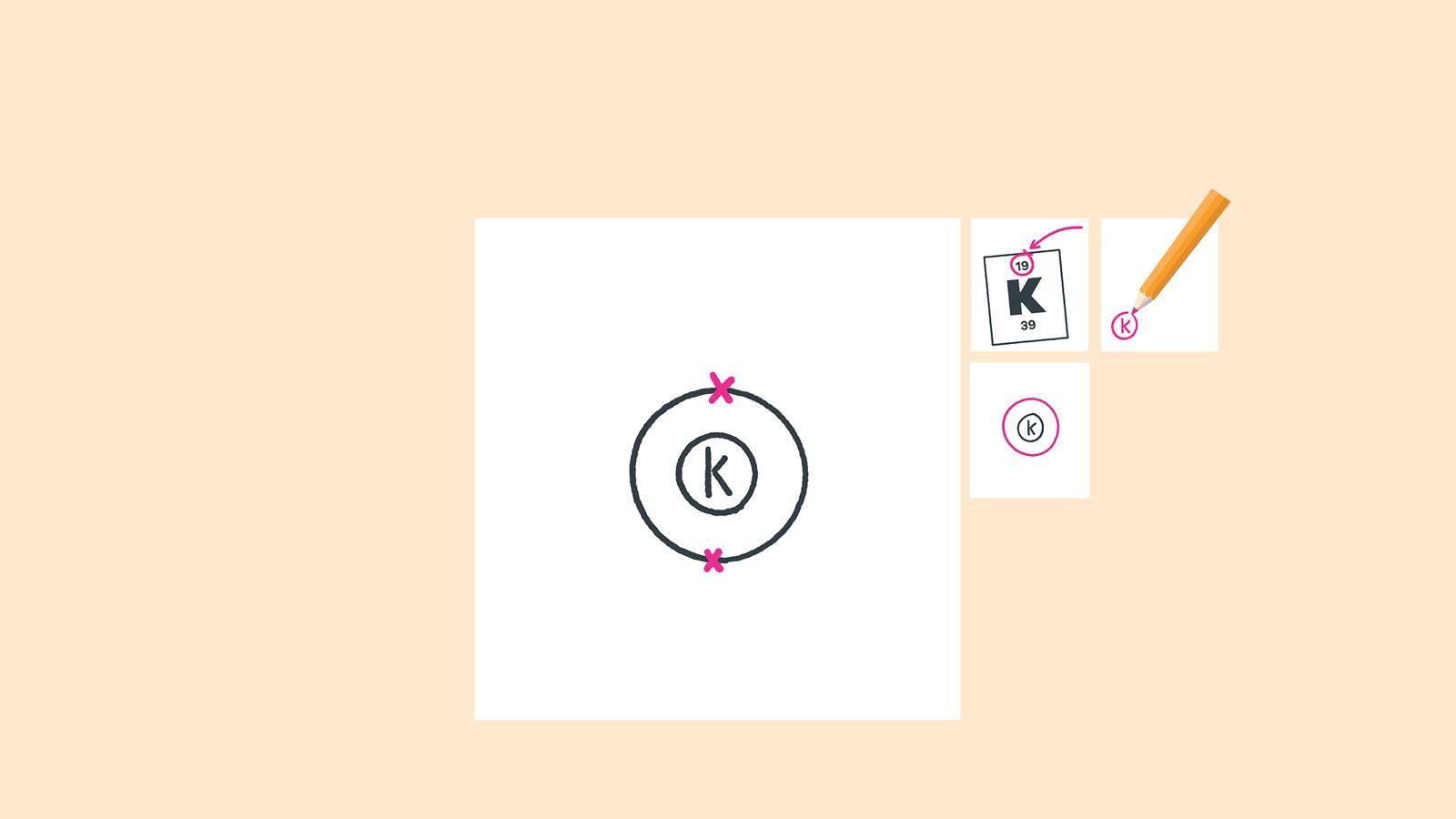
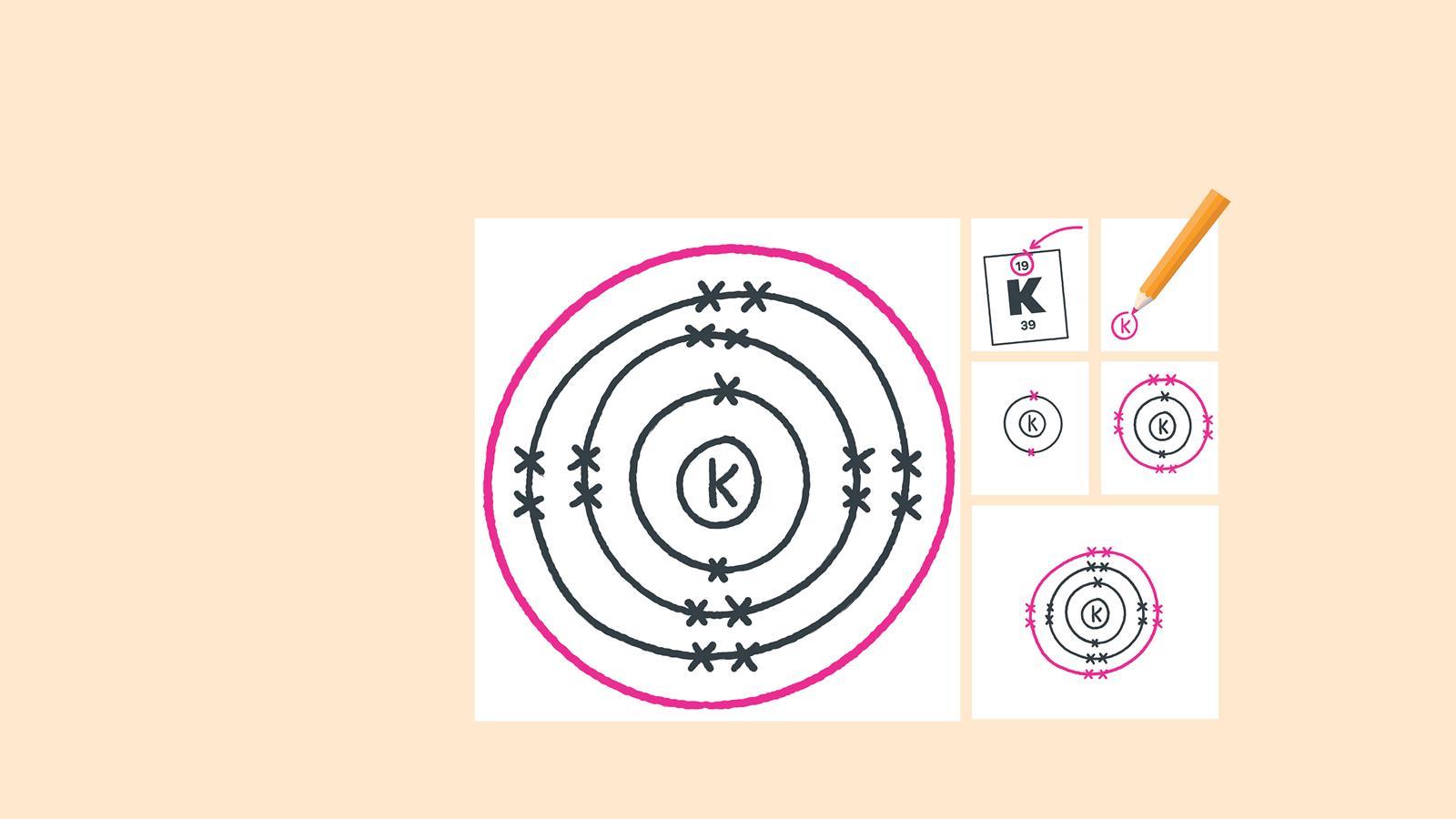
For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left.

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
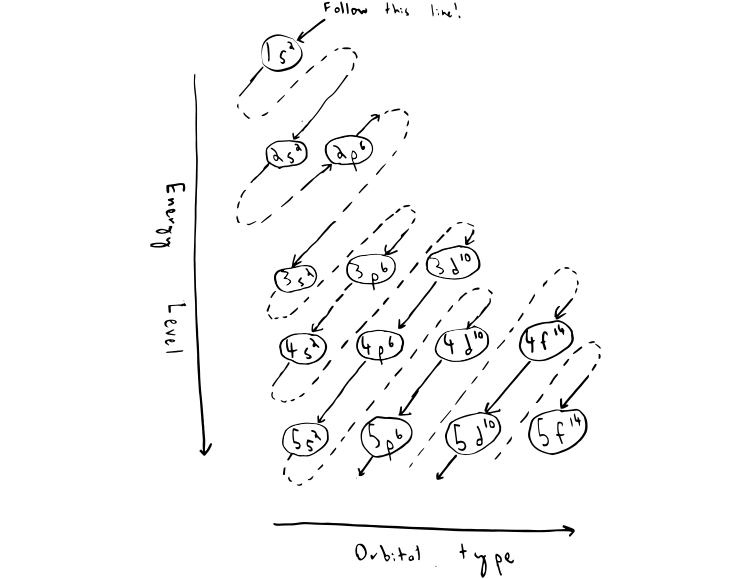
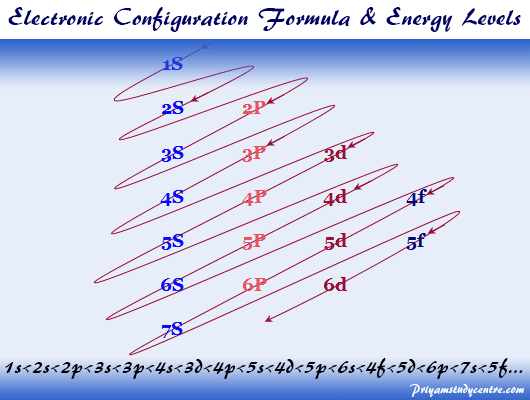
Electron Configuration Chart for All Elements in the Periodic Table. There are 118 elements in the periodic table. Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom.
Electron Configurations. The content that follows is the substance of General Chemistry Lecture 26. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements.
Electron distribution diagram of carbon. Carbon has 4 valence electrons can bond to 4 items and typically forms covalent bonds with other elements. Multiple covalent bonds the chemical context of life ppt download 9 6 quantum mechanical orbitals and electron configurations drawing electron configuration diagrams chemistry for all the. Make an ...
Transcribed image text: Some Scientists think that life elsewhere in the universe might be based on the element silicon, rather than on carbon, as on Earth. Look at the electron distribution diagram for silicon, and draw the Lewis dot structure for silicon on your note. Analyze your knowledge about it and propose a short essay about the possible silicon-based life.
The electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements contains all the elements in increasing order of atomic number.. To save room, the configurations are in noble gas shorthand.This means part of the electron configuration has been replaced with the element symbol of the noble gas symbol.
The spatial distribution of electrons occupying each of these orbitals is shown in the diagram below. Very nice displays of orbitals may be found at the following sites: J. Gutow, Univ. Wisconsin Oshkosh R. Spinney, Ohio State M. Winter, Sheffield University. The valence shell electron configuration of carbon is 2 s 2, 2p x 1, 2p y 1 & 2p z 0 ...











:max_bytes(150000):strip_icc()/carbonatom-58b602855f9b5860464c8bf6.jpg)











0 Response to "45 electron distribution diagram of carbon"
Post a Comment