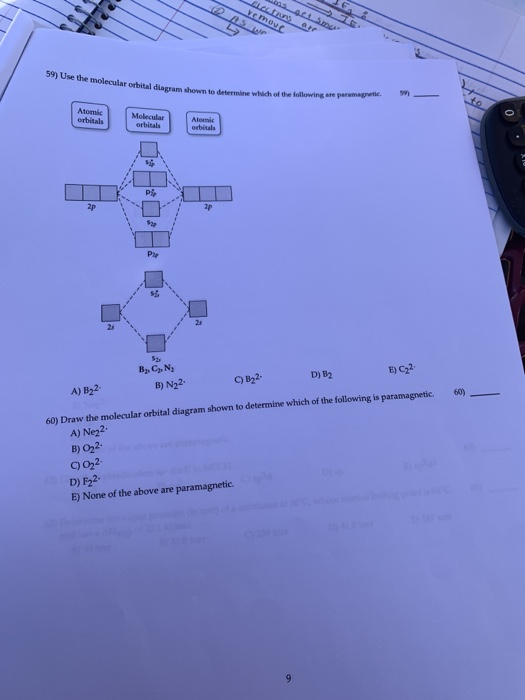
44 draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. It also explains the bonding in a number of other molecules, such as violations of the octet rule and more molecules with more complicated bonding (beyond the scope of this text) that are difficult to describe with Lewis structures. For this, we need to determine the bond order for each species. The bond order tells us the stability of a bond: a higher bond order means the bond is more stable. Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion.
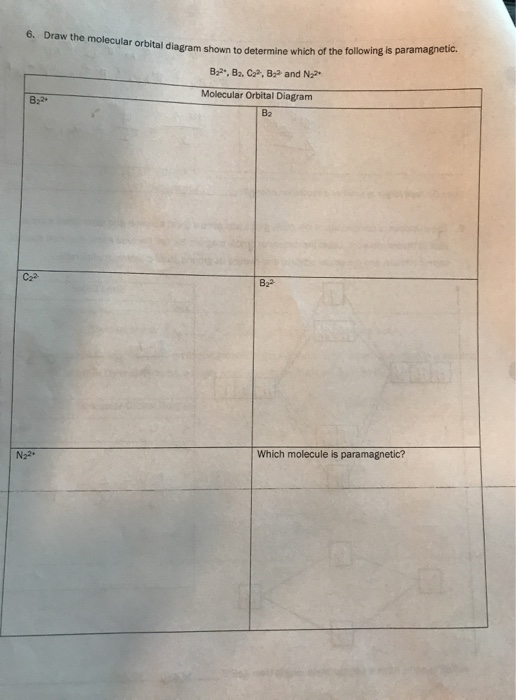
Sign In to Writing (Essays) Science. Chemistry Q&A Library Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+, B2, C22-, B22-, and N22+. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+, B2, C22-, B22-, and N22+. Start your trial now!
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
C o22 use the molecular orbital diagram shown to determine which of the following are paramagnetic. Molecular orbital diagram. Periodic trends determine. A n22 b b2 c b22 d c22 e c22. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. Molecular Orbital Theory QUEST ION 14 How many unpaired electrons are in the orbital diagram for Co2 ion? (SHOW WORK, DRAW THE ORBITAL DIAGRAM) O 0 0 1 04 O 3 7 O 6 O 5 02 QUEST ION 15 Which of the following is diamagnetic? (SHOW WORK, DRAW THE NOBLE GAS ORBITAL DIAGRAM S) O 37Rb O 15P 13A1 0 24Cr O 48Cd QUEST ION 16 Answer Draw the orbital diagram of the element. Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.Draw the molecular orbital diagram for Ne 2 + and determine if the bond between the two atoms will be stable.
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. F22+. O22-. Ne22+. O22+. None of the above are paramagnetic. Best Answer. This is the best answer based on feedback and ratings. 100% (43 ratings) A asdfasdf b asdfasdf c asdf d f2 2 e none of the above are paramagnetic. Molecular Orbital Diagram Wikipedia 1 draw the molecular orbital diagrams to determine which of the following is most stable. Draw the molecular orbital diagram shown to determine which of the following is most stable. Fill in the mo diagram that corresponds to each of ... (i) Be2 molecule: The electronic configuration of Be(Z = 4) is: 4 Be 1s 2 2s 1 Be 2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms. Number of valence electrons in Be atom = 2 Thus in the formation of Be 2 molecule, two outer electrons of each Be atom i.e. 4 in all, have to be accommodated in various molecular orbitals in the increasing order of their energies. The higher the energy the absorption, the stronger the acid-base interaction. MOLECULAR ORBITAL DIAGRAM KEY Draw molecular orbital diagrams for each of the following molecules or ions. Determine the bond order of each and use this to predict the stability of the bond. Determine whether each is paramagnetic or diamagnetic. a.
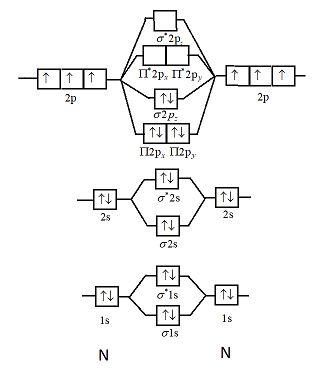
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Use molecular orbital diagram shown to determine which is most stable a o22 bf2 c f22 d f22 e ne22 a. A asdfasdf b asdfasdf c asdf d f2 2 e none of the above are paramagnetic. Label each and each and every molecular orbital with its call sigma pi and position the accessible electrons interior the perfect atomic orbitals and molecular orbitals ... Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals. Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown: (i) Electronic configuration: (ii) Bond order: Here N b = 8; N a = 4 The two oxygen atoms in a molecule of oxygen are united through two covalent ...
Also see here... Bond order for "NO"^+ Order by bond length: "NO", "NO"^(+), "NO"^(-) Is "CO" a Lewis acid? "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is already calculated in the ... E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2+ B) N2^2+ C) B2 5 2 10 10 C C 2 2 20 20 C C FIG. 2. The 2σg (left panel) and 2σu (right panel) molecular orbital s of C2 The third (in terms of increasing energy) orbital of σg symmetry is shown below. This is also a linear combination of the 2s and 2pz orbital s. There is a substantial buildup of The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
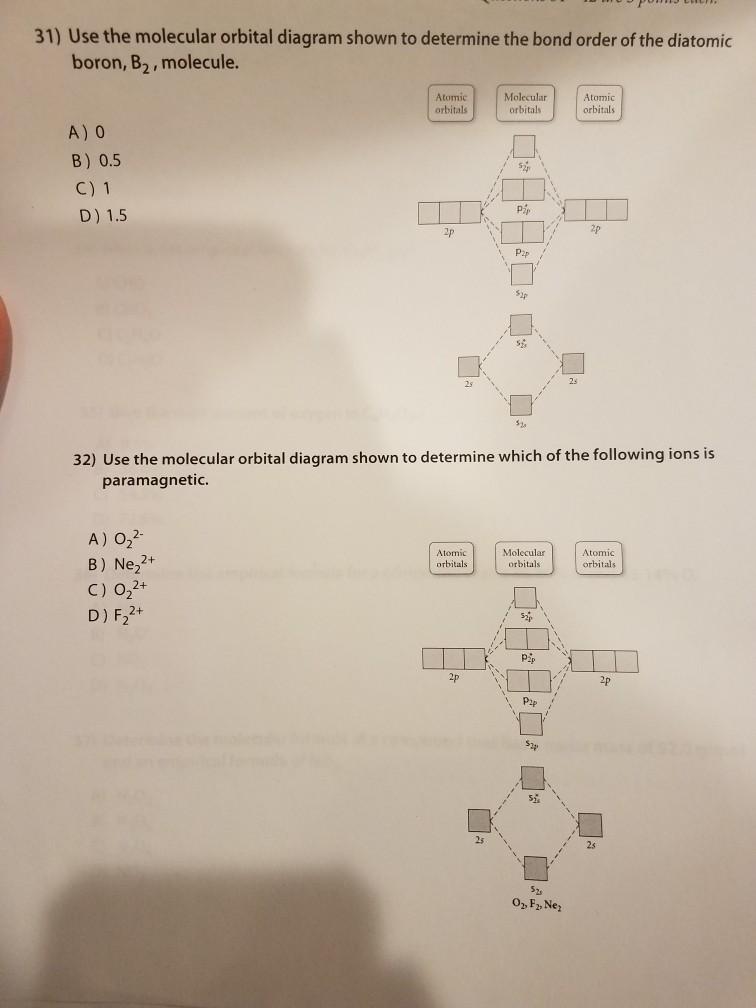
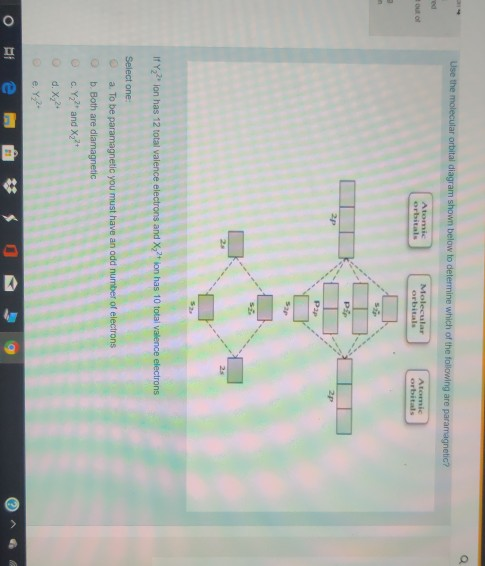
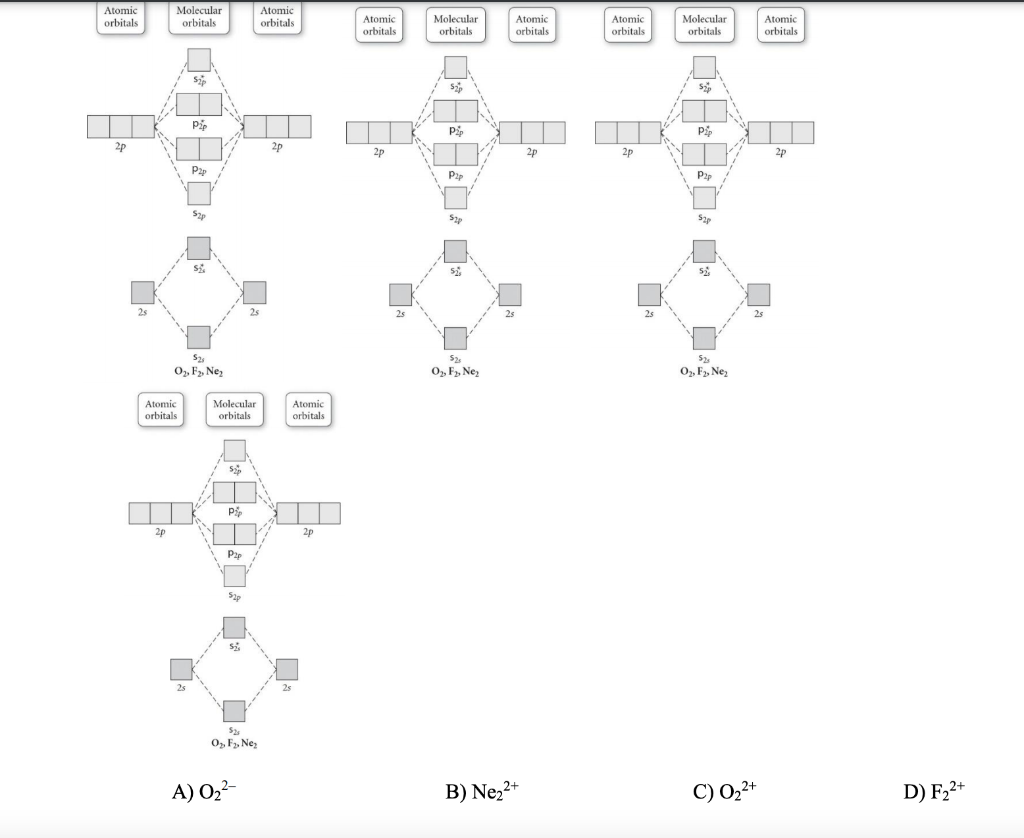
Problem: Use the molecular orbital diagram shown to determine which of the following are paramagnetic.A. Ne22+ B. O22+ C. F22+ D. O22- E. None of the above are paramagnetic. Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A. Ne 22+ B. O 22+ C. F 22+ D. O 22- E.
Answer to Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. N22+ B22+ B B2 CeV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly; this is shown in the diagram by a slight stabilization of the lowest energy ...
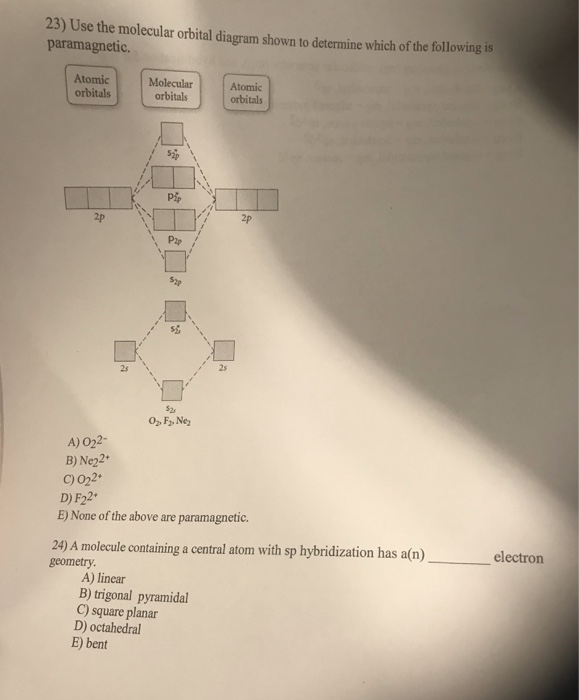
59) Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) O2^2−. B) Ne2^2+. C) O2^2+. D) F2^2+. E) None of the above are paramagnetic. D) F2^2+. 60) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2+.
be paramagnetic with a bond order of 2. The populations of the bonding (8 electrons) and antibonding (4 electrons) molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p * orbitals exhibit Cs symmetry.

Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Most Stable A Homeworklib
Determine the number and type of occupied bonding orbitals shown. sigma molecular orbitals 2 pi molecular orbitals 2 Determine the number and type of occupied antibonding orbitals shown. sigma molecular orbitals 1 pi molecular orbitals 1 3/13 11/22/21, 5:37 PM Practice#12 F21- MOs - CH 101, section 009, Fall 2021 | WebAssign (c) Determine the ...

Consider The Molecular Orbital Diagram For The Ion O 2 2 Predict The Bond Order A 3 0 B 2 5 C 1 0 D 2 0 E 1 5 Consider The Following Statements Will The Ion Be Paramagnetic Or Study Com
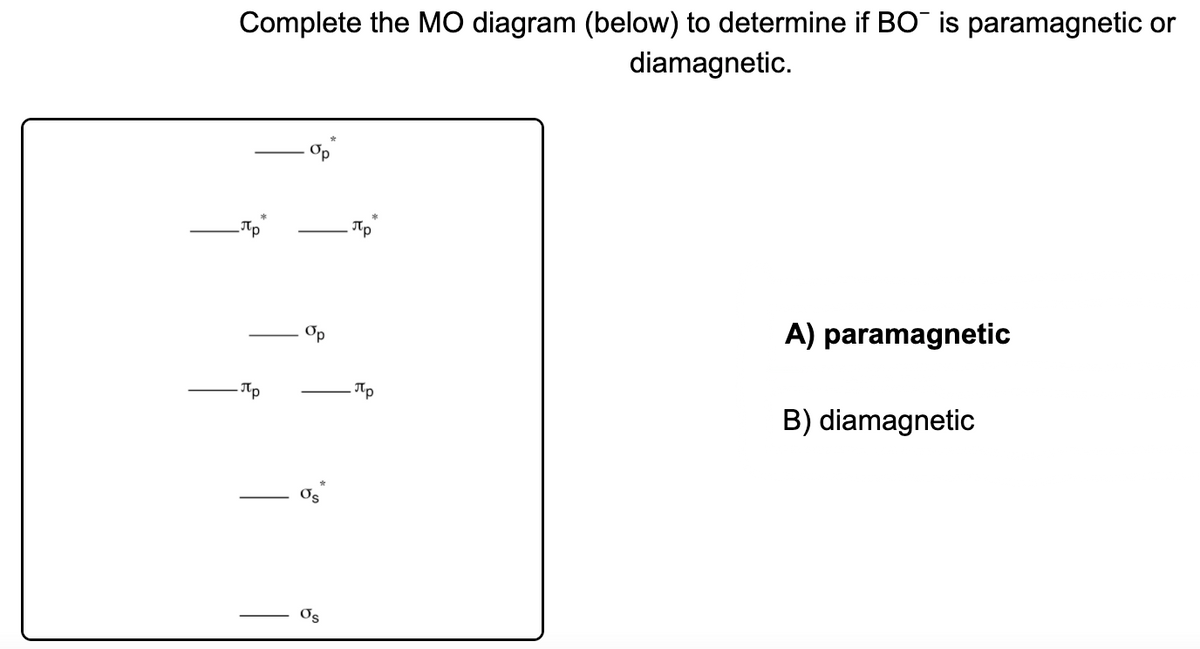
Draw the best Lewis structure for BrO4⁻ and determine the formal charge on bromine. A) -1 B) +1 ... Draw the molecular orbital diagram shown to determine which of the following is most stable. A) F2 B) F22⁺ ... Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) O22⁻ ...
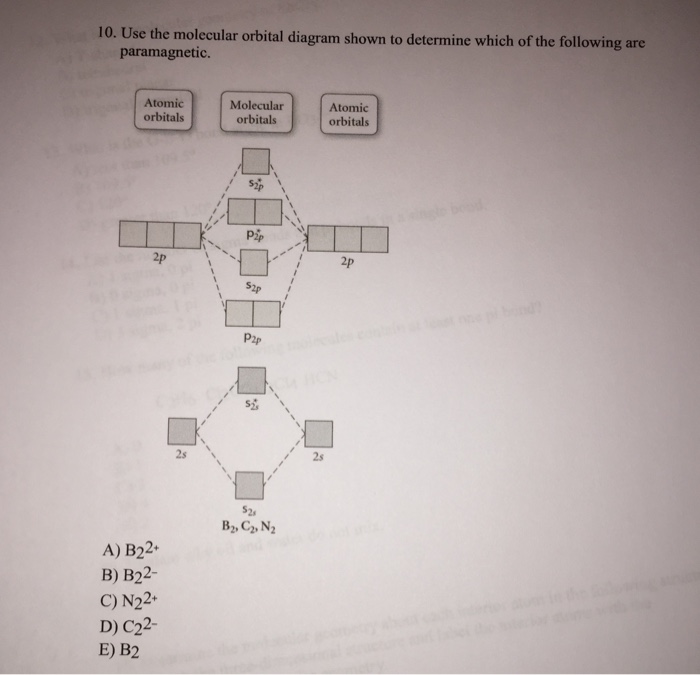
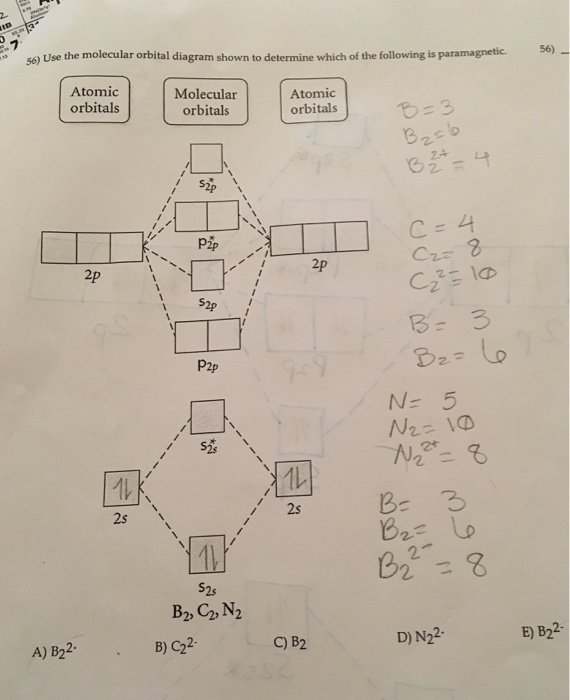
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.Answer options:B2B22+N22+C22-B22-Question: Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.Answer options:B2B22+N22+C22-B22-
Use the molecular orbital diagram shown to determine which of the following is paramagnetic. asked Jul 15, 2019 in Chemistry by brittanyr9777. general-chemistry. Nitrogen can lose an electron to form N2+. Given the molecular orbital configuration of N2 [core] (σ2s)2 (σ *2s)2 (π2p)4 (σ2p)2 is N2+ diamagnetic or paramagnetic? asked Jun 30 ...
pollutants. A molecular orbital diagram of this species is shown below. Core orbitals are omitted. Marks 8 Using arrows to indicate electrons with their appropriate spin, indicate on the above diagram the ground state occupancy of the atomic orbitals of O and H, and of the molecular orbitals of OH. In the provided boxes on the above diagram ...

Draw The Molecular Orbitals For Co In Order Of Energy And Fill Them With The Appropriate Number Of Electrons Label The Orbitals The Best You Can As Sigma Or Pi And As
a. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B_2^2+, B2, C_2^2-, B_2^2- and N_2^2+ b. Draw the Lewis structures and molecular orbital diagrams ...
Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.Draw the molecular orbital diagram for Ne 2 + and determine if the bond between the two atoms will be stable.
QUEST ION 14 How many unpaired electrons are in the orbital diagram for Co2 ion? (SHOW WORK, DRAW THE ORBITAL DIAGRAM) O 0 0 1 04 O 3 7 O 6 O 5 02 QUEST ION 15 Which of the following is diamagnetic? (SHOW WORK, DRAW THE NOBLE GAS ORBITAL DIAGRAM S) O 37Rb O 15P 13A1 0 24Cr O 48Cd QUEST ION 16 Answer Draw the orbital diagram of the element.

Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Are Paramagnetic Atkinsjewelry
C o22 use the molecular orbital diagram shown to determine which of the following are paramagnetic. Molecular orbital diagram. Periodic trends determine. A n22 b b2 c b22 d c22 e c22. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. Molecular Orbital Theory
Consider The Following Molecules No No And No Using The Molecular Orbital Theory How Do You Evaluate Them In Terms Of Bond Energy And Stability Quora

Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Are Paramagnetic Atkinsjewelry

Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic N22 B22 Homeworklib
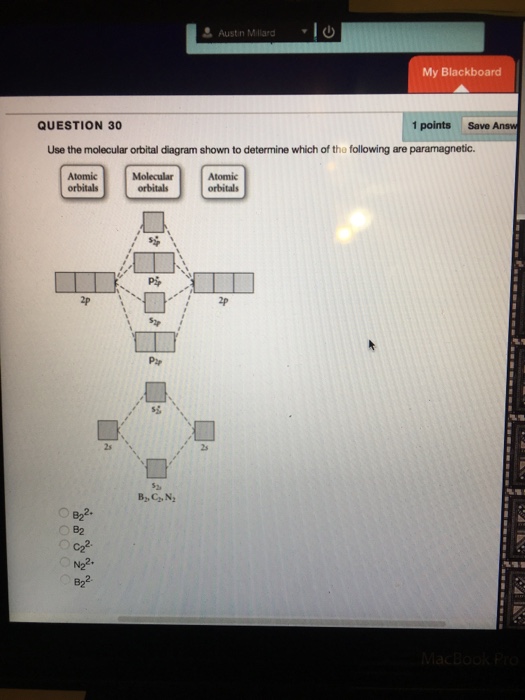
Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Are Paramagnetic Atkinsjewelry

Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic Study Com

Construct A Molecular Orbital And Energy Splitting Diagram Of The Bonding In Lih Is It Favorable Study Com

Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Are Paramagnetic Atkinsjewelry

















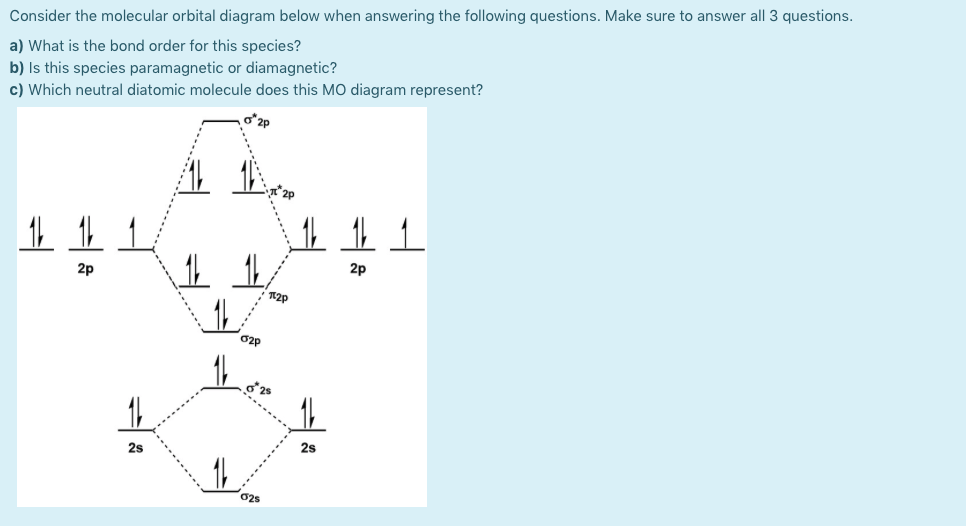

0 Response to "44 draw the molecular orbital diagram shown to determine which of the following is paramagnetic."
Post a Comment