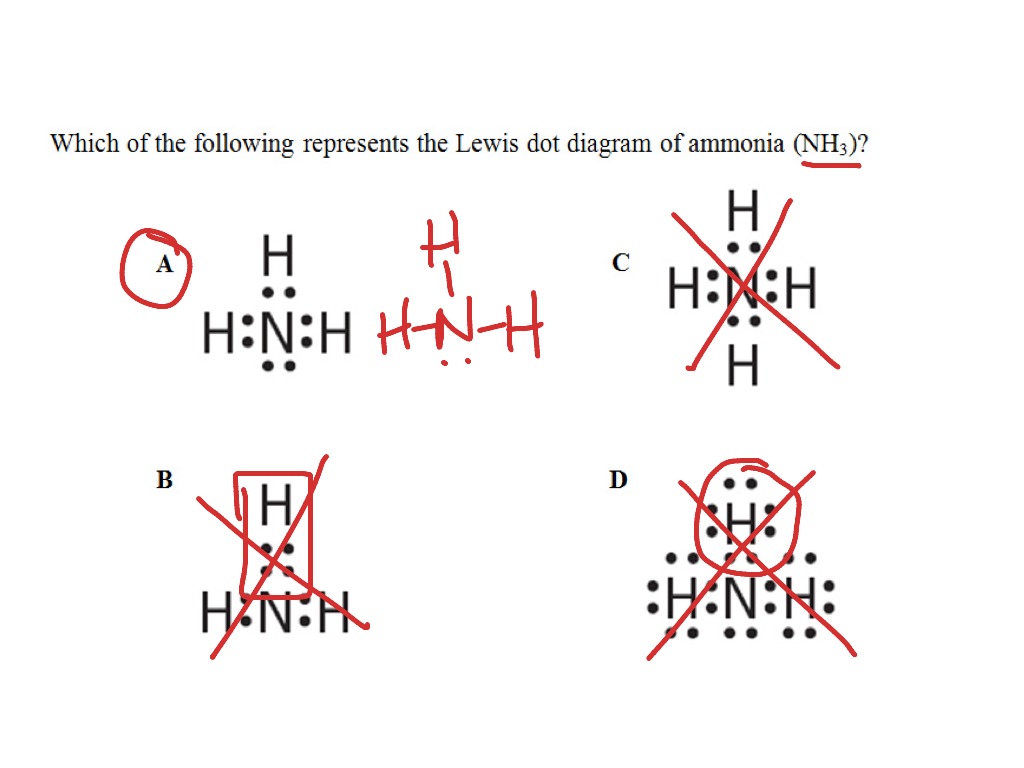
42 lewis dot diagram of ammonia
The lewis dot structure for nh3 starts with an n atom connected on three sides with a dash each to an h atom. Molecular structures are lewis dot structures example. Nh 3 ammonia is a commonly tested lewis structure. There are eight valence electrons in ammonia. Drawing the lewis structure for nh 3. Ammonia has the formula nh3. 14+ Electron Dot Structure Of Nh3. The ammonia molecule has one unshared pair of. The best way to figure this out is to draw the lewis structure. Then, using the aufbau principle, draw electron dot structures for each of the elements. This is the reason why ammonia acts as a lewis base, as it can donate those electrons.
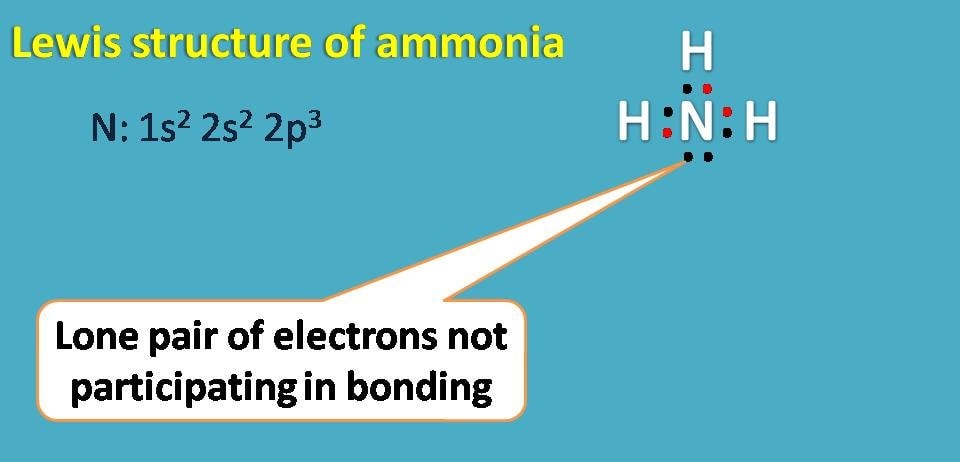
Lewis Structure (electron dot diagram) for ammonia OR . Note that there are 3 covalent bonds (3 bonding pairs of electrons) in total, and that there is a lone pair (non-bonding pair) of electrons on the nitrogen atom.


Lewis dot diagram of ammonia
Match. Gravity. Which of the following correctly describes a Lewis dot structure for carbon? Click card to see definition 👆. Tap card to see definition 👆. C with one dot on each of the four sides of the C. Click again to see term 👆. Tap again to see term 👆. What do Lewis structures show? Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. The Lewis Dot Structure for NH3. Created by MakeTheBrainHappy. The Lewis Dot Structure for NH3 (Ammonia) is shown above. You could also represent the bonds as dots between the two atoms, but this may be confused with the lone pair electrons on the nitrogen. Each atom in the bond has a full valence shell, with nitrogen having access to eight ...
Lewis dot diagram of ammonia. NH3 Lewis Structure, Geometry, and Hybridization. Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state. Ammonia is lighter than the air, colorless, and pungent in smell. Is ammonia dot and cross diagram? An ammonia molecule, NH 3, forms when one nitrogen atom shares its outer electrons with three hydrogen atoms. There are two types of dot and cross diagram - one without circles, and one with. What is the Lewis dot structure of c2h4? In the lewis structure of C2H4, there are only four C-H bonds, one C=C bond ... A simple method for drawing the Lewis structure for ammonia. Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer.It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3.. Video: Drawing the Lewis Structure for NH 3
Answer the following questions about nitrogen, hydrogen, and ammonia. (a) In the boxes below, draw the complete Lewis electron-dot diagrams for N 2 and NH 3. The correct structures are shown in the boxes above. Two points are earned for the correct Lewis electron-dot diagrams (1 point each). The former, known as a 'Lewis dot diagram,' indicates a pair of shared electrons between the atomic symbols, while the latter, known as a 'Lewis structure,' uses a dash to indicate the pair of shared electrons that form a covalent bond. More complicated molecules are depicted this way as well. Electron dot diagram for nh3. Ammonia nh 3 is a commonly tested lewis structure due to its widespread use in agriculture as a fertilizerit also is a good example of a molecule with a trigonal prymidal molecular geometry. It has one valence electron but we have 3 hydrogens so lets mutiply that by 3. Learn vocabulary terms and more with ... What is the Lewis dot structure for ammonia? In the lewis structure of ammonia (NH3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Can you draw the electron dot structure of […]
Alternate lewis dot structure of water. 10+ Nh3 Lewis Structure. This is the reason why ammonia acts as a lewis base, as it can donate those electrons. (1) you have two chlorine atoms. Bonding and structure in covalent compounds. Electron dot structures or lewis dot formula can be drawn if the molecular formula of the compound is known. A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale... Starting with the Lewis dot structure of Ammonia, Nitrogen has 5 valence electrons and each hydrogen has 1 valence electron. So, the total valence electrons are 8. Hydrogen always goes on the outside, so Nitrogen is the central atom. After the three valence electrons of Nitrogen have bonded with three Hydrogens, we still have two valence ... The Lewis structure of ammonia, #NH_3#, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom.This is the reason why ammonia acts as a Lewis base, as it can donate those electrons.
Similarly, you may ask, what is the Lewis dot structure for nh3? Explanation: The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons.
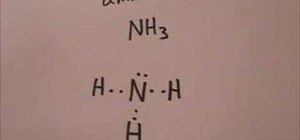
The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3.
A video explanation of how to draw the Lewis Dot Structure for Ammonia, along with information about the compound including Formal Charges, Polarity, Hybrid ...
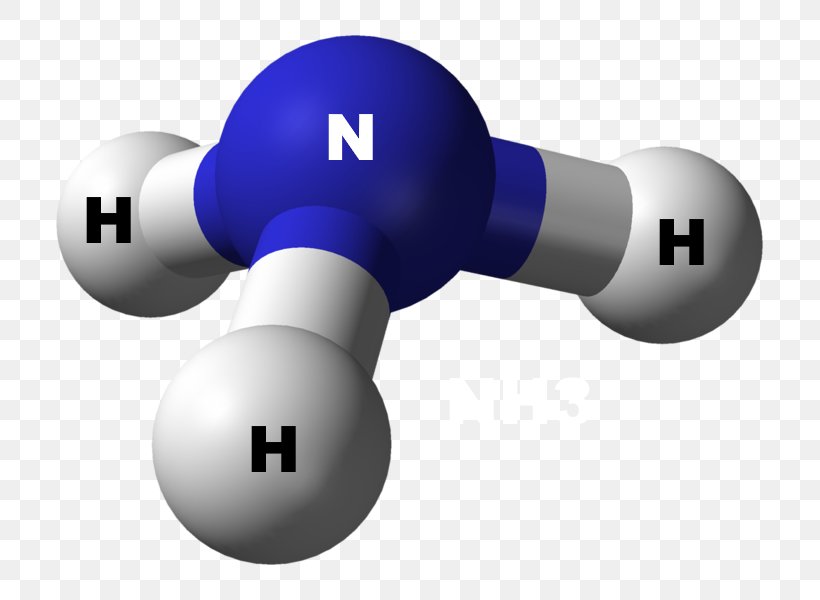
Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride formed of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can form an NH4+ ion by accepting a proton. In this blog post, we will learn about the Lewis dot structure, electron geometry, and molecular geometry of this molecule.
This chemistry video tutorial explains how to draw the lewis structure of NH3 also known as Ammonia.My Website: https://www.video-tutor.netPatreon: https:/...
The electron-dot structure of NH3 places one pair of nonbonding electrons in the valence shell of the nitrogen atom. This means that there are three bonded atoms and one lone pair for a coordination number of four around the nitrogen, the same as occurs in H2O. The Lewis dot structure for ammonia, NH3. Secondly, what is the shape of nh3 ...
Hey everyone, welcome to the Mentor Center! In today's video, I draw out the Lewis dot structure for NH3, commonly known as ammonia.👍 Like 📽️ Subscribe ...
NH4+ Lewis Structure - How to Draw the Dot Structure for NH4+ (Ammonium ... | Molecular geometry, Chemistry, Science chemistry
Lewis dot structure of ammonia. Alternatively a dot method can be used to draw the lewis structure of NH3. Calculate the total valence electrons in NH3 molecule. N=5,H=1x3=3 Total=8 Put Nitrogen in the center and three hydrogen atoms on the sides. Lewis Dot Structures (2): Water and Ammonia.
Here, we will be using the determined total number of valence electrons per atom and drawing them in the proper places. Reference the "How to Draw a Lewis Dot Structure" for a Step by Step guide. See the following Lewis dot structure diagrams for a few covalent compounds. Example 1. Ammonia, NH 3
Drawing the Lewis Structure for NH 3. Viewing Notes: NH 3 (Ammonia) is a commonly tested Lewis structure. It's not particularly difficult but is an important structure. In the NH 3 Lewis structure (and all structures), hydrogen goes on the outside. Remember, too, that hydrogen only needs two valence electrons to have a full outer shell.
The Lewis Dot Structure for NH3. Created by MakeTheBrainHappy. The Lewis Dot Structure for NH3 (Ammonia) is shown above. You could also represent the bonds as dots between the two atoms, but this may be confused with the lone pair electrons on the nitrogen. Each atom in the bond has a full valence shell, with nitrogen having access to eight ...
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Match. Gravity. Which of the following correctly describes a Lewis dot structure for carbon? Click card to see definition 👆. Tap card to see definition 👆. C with one dot on each of the four sides of the C. Click again to see term 👆. Tap again to see term 👆. What do Lewis structures show?













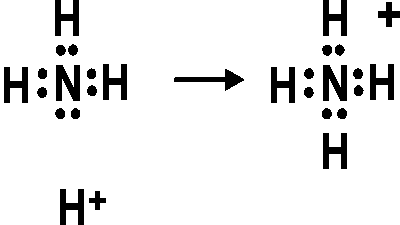























0 Response to "42 lewis dot diagram of ammonia"
Post a Comment